Question
Question: In the reaction, The major product $A$ is ...
In the reaction,
The major product A is
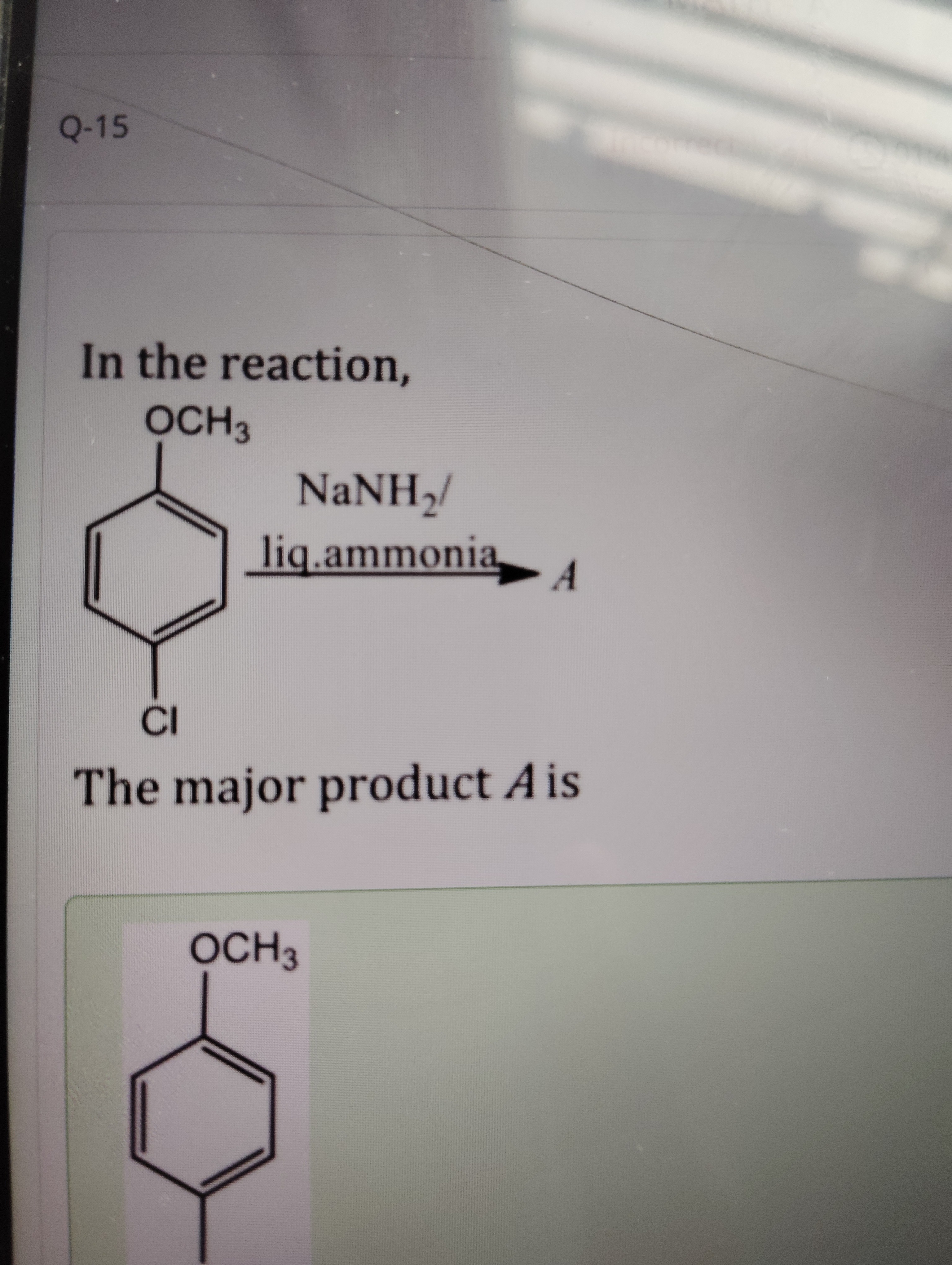
4-methoxyaniline
Solution
The reaction involves 1-chloro-4-methoxybenzene (p-chloroanisole) reacting with sodium amide (NaNH₂) in liquid ammonia (liq. ammonia). This set of reagents is characteristic for a nucleophilic aromatic substitution reaction proceeding via the benzyne mechanism.
The mechanism involves three main steps:
-
Formation of the benzyne intermediate (Elimination): The strong base, amide ion (NH₂⁻), abstracts an acidic proton ortho to the halogen (Cl). There are two possible ortho positions to the chlorine atom (C1): C2 and C6. The methoxy group (-OCH₃) is at C4.
- Abstraction of H from C2: This forms a carbanion at C2. C2 is meta to the -OCH₃ group. The resonance (+M) effect of -OCH₃ does not significantly affect the meta position, and its inductive (-I) effect (electron-withdrawing) can slightly stabilize the carbanion.
- Abstraction of H from C6: This forms a carbanion at C6. C6 is ortho to the -OCH₃ group. The strong electron-donating resonance (+M) effect of -OCH₃ would destabilize a carbanion at the ortho position.
Therefore, the abstraction of the proton from C2 is kinetically favored, leading to a more stable carbanion at C2. This carbanion then eliminates the chloride ion (Cl⁻) from C1 to form the benzyne intermediate with a triple bond between C1 and C2. This intermediate is 4-methoxybenzyne (considering OCH₃ at C4).
-
Nucleophilic attack on the benzyne (Addition): The amide ion (NH₂⁻) acts as a nucleophile and attacks one of the carbons of the benzyne triple bond.
For 4-methoxybenzyne (triple bond between C1 and C2, OCH₃ at C4):
- Attack at C1 (original Cl position): The triple bond shifts, forming a carbanion at C2. C2 is meta to the -OCH₃ group.
- Attack at C2 (ortho to original Cl position): The triple bond shifts, forming a carbanion at C1. C1 is para to the -OCH₃ group.
The -OCH₃ group is an electron-donating group by resonance (+M effect). It destabilizes negative charges at ortho and para positions. Thus, the carbanion formed at C2 (meta to -OCH₃) is more stable than the carbanion formed at C1 (para to -OCH₃).
Therefore, the nucleophilic attack at C1 is preferred, leading to the carbanion at C2.
-
Protonation of the carbanion: The carbanion formed in the previous step (at C2) abstracts a proton from liquid ammonia (the solvent) to form the final product.
The final product, A, has the -NH₂ group at the original position of the chlorine atom (C1) and the -OCH₃ group at C4. This compound is 4-methoxyaniline (also known as p-anisidine).
Structure of Reactant (1-chloro-4-methoxybenzene):
OCH3
|
C4
/ \
C3 C5
/ \
C2 C6
| |
C1-------C
|
Cl
Structure of Major Product (4-methoxyaniline):
OCH3
|
C4
/ \
C3 C5
/ \
C2 C6
| |
C1-------C
|
NH2
The image provided in the question shows the major product A as:
OCH3
|
C
/ \
C C
/ \
C C
| |
C---------C
|
NH2
This corresponds to 4-methoxyaniline.
The final answer is 4-methoxyaniline
Explanation of the solution:
The reaction of 1-chloro-4-methoxybenzene with NaNH₂/liq. ammonia proceeds via the benzyne mechanism. The strong base abstracts the ortho-proton at C2 (meta to -OCH₃) preferentially over C6 (ortho to -OCH₃) due to carbanion stability. This forms 4-methoxybenzyne. The nucleophile (NH₂⁻) then attacks C1 (para to -OCH₃) of the benzyne, as this leads to a more stable carbanion at C2 (meta to -OCH₃). Subsequent protonation yields 4-methoxyaniline as the major product.
