Question
Question: Least Aridic Strength...
Least Aridic Strength
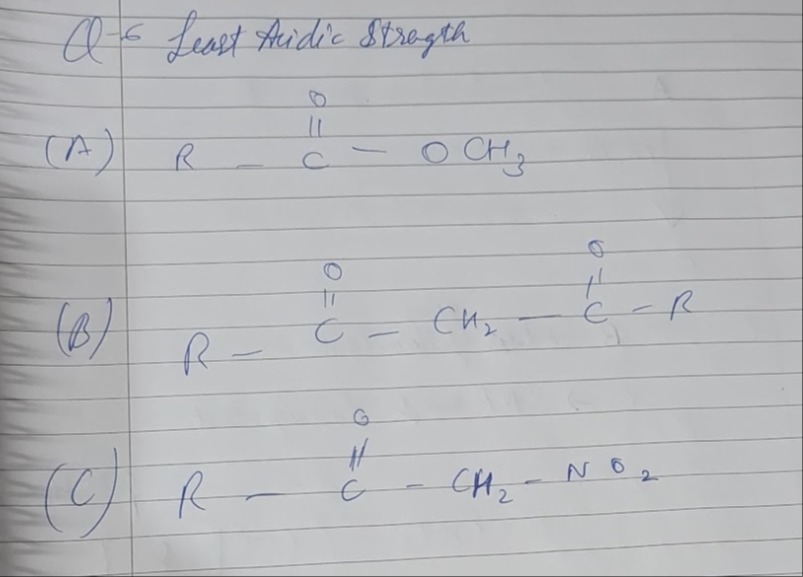
R- ∣∣CO - OCH3
R- ∣∣CO-CH2-∣∣CO-R
R-∣∣CO-CH2-NO2
R- ∣∣CO - OCH3
Solution
Acidity depends on the stability of the conjugate base.
-
Compound (A) (Ester): Alpha-hydrogens are deprotonated. The resulting carbanion is stabilized by resonance with one carbonyl group. Esters are relatively weak acids (pKa ~25).
-
Compound (B) (β-diketone): The methylene protons between two carbonyl groups are deprotonated. The resulting carbanion is highly stabilized by resonance with both carbonyl groups, delocalizing the negative charge over two oxygen atoms. β-diketones are moderately strong acids (pKa ~9-11).
-
Compound (C) (Nitro-ketone): The methylene protons between a carbonyl and a nitro group are deprotonated. The resulting carbanion is very strongly stabilized by resonance with both the carbonyl and the highly electron-withdrawing nitro group, delocalizing the negative charge over the carbonyl oxygen and two nitro oxygens. Nitro-ketones are very strong acids (pKa ~4-6).
Comparing the stability of the conjugate bases: (C) > (B) > (A).
Therefore, the order of acidic strength is (C) > (B) > (A).
The least acidic compound is (A).
