Question
Question: Pyrolysis of ethyl acetate gives: A. \[C{H_3}COC{H_3}\] B. \[C{H_2} = C{H_2}\] C. \[C{H_2} = ...
Pyrolysis of ethyl acetate gives:
A. CH3COCH3
B. CH2=CH2
C. CH2=C=O
D. CH3−CHO
Solution
Pyrolysis is the thermal decomposition of a substance at a particular temperature in an inert atmosphere, it involves a change of chemical composition. On decomposition of ester carboxylic acid and ethylene are formed.
Complete step by step answer:
Pyrolysis of ethyl acetate (CH3COOCH2CH3) gives ethane and acetic acid.
(ethyl acetate)(CH3COOCH2CH3)ΔPyrolysis(acetic acid)CH3COOH+(ethylene)CH2= CH2
Ethyl acetate is heated in the presence of liquid nitrogen and glass wool.
Pyrolysis reaction is converting esters containing a β−hydrogen atom into the corresponding carboxylic acid and alkene. The reaction is a unimolecular elimination and operates in a syn-elimination. The mechanism involves a unimolecular six – centered transition state producing equimolar ethylene and acetic acid.
Mechanism of pyrolysis of ethyl acetate
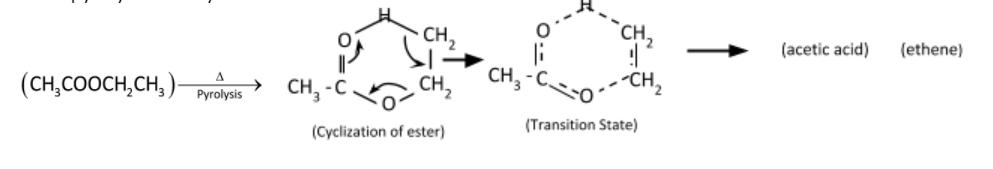
Therefore, the correct option is (D).
Note:
Ethyl acetate is a colorless liquid and is commonly known as ethyl ethanoate. Ethyl acetate is a polar aprotic solvent and it is a standard solvent. It is an important chemical compound. It is used especially for paints, varnishes, lacquers, cleaning and perfumes. The thermal decomposition of ethyl acetate follows a first order unimolecular reaction. The kinetic and thermodynamic parameters are obtained experimentally by applying the Arrhenius equation at a temperature range from 400∘ to 600∘C. The products from the pyrolysis of ethyl acetate were analyzed by gas chromatography.
