Question
Question: Pyridine is less basic than triethylamine because: A. Pyridine has aromatic character B. Nitroge...
Pyridine is less basic than triethylamine because:
A. Pyridine has aromatic character
B. Nitrogen in pyridine is sp2 hybridised
C. Pyridine is a cyclic system
D. In pyridine, lone pair of nitrogen is delocalised
Solution
Hint: Pyridine is an aromatic compound. The structure of pyridine is similar to the structure of benzene in which a carbon atom is replaced by a nitrogen atom.
Complete step by step answer:
Pyridine is a heterocyclic organic compound with the chemical formula C5H5N. Pyridine is structurally related to benzene, with one methine group replaced by a nitrogen atom. Pyridine is actually colourless but some older and impure samples can appear yellow. In earlier days, pyridine was produced from coal tar.
The structure of pyridine is:
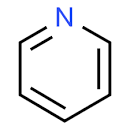
Pyridine is an aromatic cyclic structure in which the lone pair of electrons are not involved in resonance. Hence, the lone pair of electrons are not delocalised, but localised. The reason due to which pyridine is less basic than triethylamine is the presence of sp2 nitrogen in the structure of pyridine. The sp2 nitrogen is more electronegative than the sp3 nitrogen. Hence, the tendency of nitrogen to attract the electrons is more which reduces its electron donating nature to foreign species.
Triethylamine, on the other hand, has sp3 nitrogen as the central atom. Also, the ethyl groups increase the electron density on nitrogen, which in turn increases the electron donating ability of the central atom nitrogen. The structure of triethyl amine is:
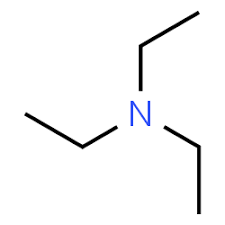
Hence, pyridine is less basic than triethylamine due to the presence of sp2 nitrogen.
Therefore, the correct answer is (B).
Note: Remember that the sp2 carbons in the structure of pyridine also reduce the electronic donating ability of nitrogen. Triethyl amine has only sp3 carbons.
