Question
Question: Propyne molecule contains: A. 6 sigma and 2 pi bonds B. 5 sigma bonds C. 5 pi bonds and 1 sigm...
Propyne molecule contains:
A. 6 sigma and 2 pi bonds
B. 5 sigma bonds
C. 5 pi bonds and 1 sigma bond
D. 2 sigma and 3 pi bonds
Solution
If the covalent bond in two different chemicals is one means it contains one sigma bond. If a double bond is present in between different elements means it contains one sigma bond and one pi bond. If a triple bond is present in between different elements means it contains one sigma and two pi bonds.
Complete step by step answer:
- The given molecule in the question is propyne.
- To know about the number of sigma bonds and number of pi bonds in any molecule first we should know the structure of the molecule.
- The structure of the propyne is as follows.
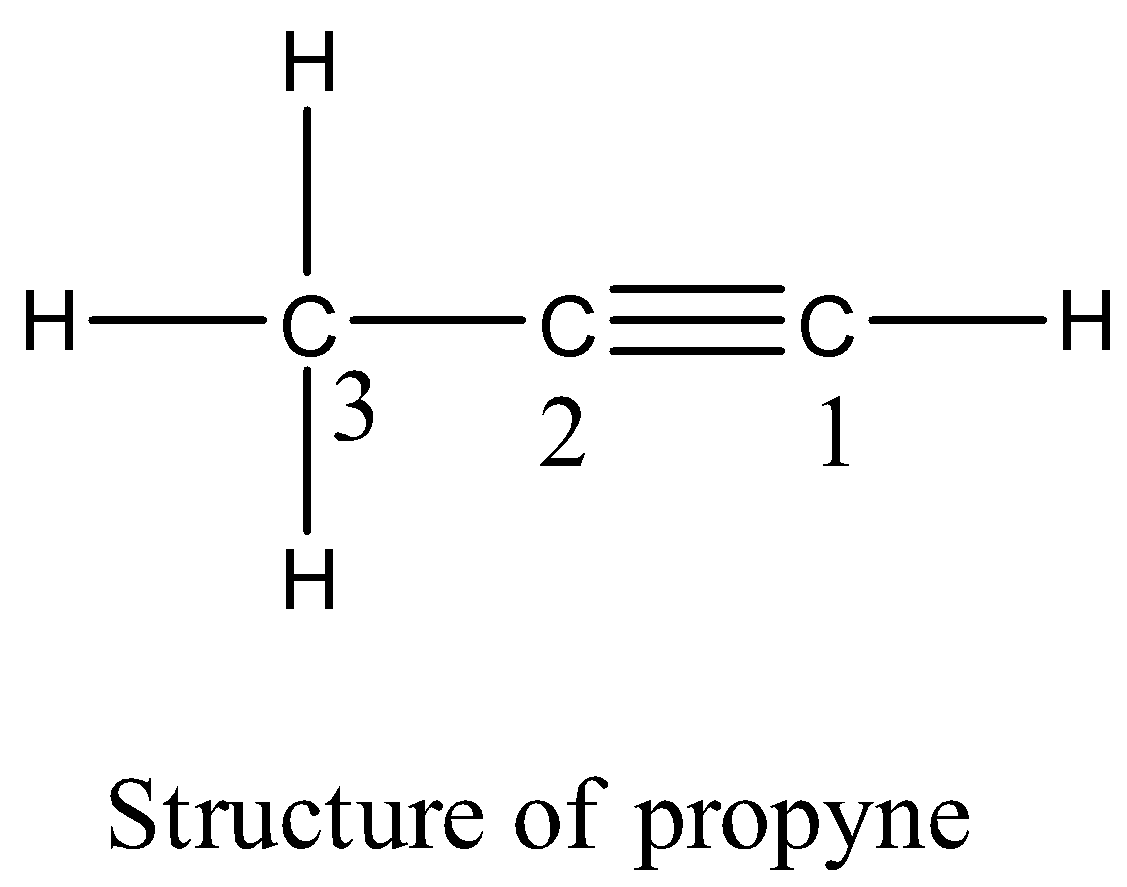
- Propyne contains three carbon atoms and four hydrogen atoms in the above structure.
- Carbon-1 is attached to one hydrogen atom through a single bond (sigma bond) and carbon-1 is attached to carbon-2 through a triple bond (one sigma bond and two pi bonds).
- Carbon-2 is attached to carbon-3 through a single bond (sigma bond).
- Carbon-3 is attached to three hydrogens through three bonds (three sigma bonds).
- Therefore, the total number of sigma bonds is 6 and the total number of pi bonds is two present in the structure of the propyne.
- So, the correct option is A.
Note: A sigma bond is going to form in between two atoms by axial overlapping of the orbitals of the atoms and a pi bond is going to form in between two atoms by side wise overlapping of the orbitals of the atoms.
