Question
Question: Propene \[C{{H}_{3}}-CH=C{{H}_{2}}\]can be converted into 1-propanol by oxidation. Indicate which ...
Propene CH3−CH=CH2can be converted into 1-propanol by oxidation.
Indicate which sets of reagents amongst the following is ideal to effect the above conversion?
(a)- KMnO4 (alkaline)
(b)- Osmium tetroxide (OsO4/CH2Cl2)
(c)- B2H6 and alk H2O2
(d)- O3 and Zn/H2O
Solution
Hint: Herbert C. Brown developed the hydroboration technique, mostly carried out at Purdue University and won the Nobel Prize in 1979 for his invention. It paved a new way to practice organic chemistry.
Complete step by step solution:
Hydroboration-oxidation reaction is used to convert alkene into alcohol. In this reaction, borane from the borane compound gets added across the carbon‐carbon double bond of the alkene. In other words, the addition of borane will happen at the double bond in such a manner that the boron atom gets attached to the sp2 carbon carrying a greater number of hydrogen atoms. As a result, an organoboron compound (trialkyl borane) is formed.
Again, this formed organoboron compound reacts with hydrogen peroxide in a basic medium and oxidizes to alcohol by the addition of water to the alkene in a way opposite to Markovnikov's rule. This results in regiochemistry of this reaction following anti-Markovnikov’s rule, i.e., hydrogen goes to the more substituted carbon atom of the alkene.
Stereochemistry of this reaction is syn, i.e. boron and hydrogen are on the same side of the product.
The reaction can be given as:
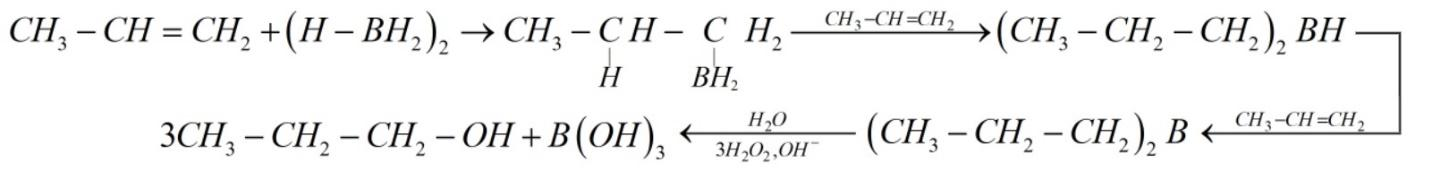
So, the correct option is (c).
Note: The boron byproduct formed in the reaction depends on the number of equivalents of the boron compound used relative to the alkene. The reaction will be the same if BH3 is used. BH3-THF is the same as BH3. Tetrahydrofuran (THF) is merely a solvent. B2H6 is another form of BH3. It behaves the same way as BH3.
