Question
Question: Propanoic acid occurs naturally as a result of the bacterial fermentation of milk and is partly resp...
Propanoic acid occurs naturally as a result of the bacterial fermentation of milk and is partly responsible for the flavour of Swiss cheese.
Which starting materials can be used to produce propanoic acid?
1. CH3CH2CH2OH
2. CH3CH2CHO
3. CH3CH2CN
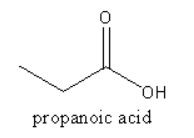
A. 1,2 and 3 are correct.
B. 1 and 2 only are correct.
B. 2 and 3 only are correct.
D. 1 only is correct.
Solution
The given reactant contains alcohol, aldehyde, and nitrile, functional group. We will convert them into carboxylic acid by using oxidizing agents. Oxidizing agents cause oxidation hence converts alcohol, aldehyde, and nitrile functional groups into a carboxylic acid.
Complete answer:
We have to prepare propanoic acid from alcohol, aldehyde, and nitrile functional groups. The conversion of alcohol, aldehyde and nitrile into acid is known as oxidation. For oxidation, strong oxidizing agents are required.
The oxidation reaction are shown as follows:
1. Potassium permanganate converts alcohol into acid.
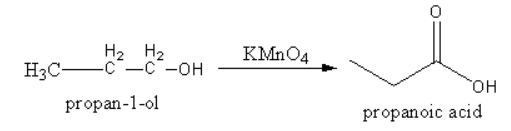
2. Chromic acid converts aldehyde into acid.
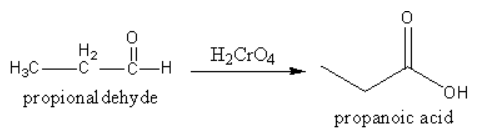
3. By simple hydrolysis nitrile can be converted into acid.
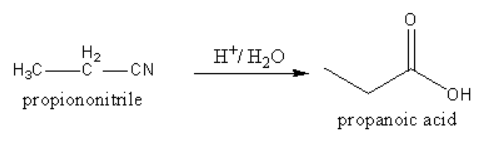
So, propanol, propionaldehyde, propionitrile all three can be converted into acid.
**Therefore, option (A) 1,2 and 3 are correct, is correct.
Additional information: **
The substances which cause oxidation are known as oxidation agents. . In place of chromic acid and potassium permanganate some other oxidizing agent can be used such as a solution of chromic acid with sulphuric acid CrO3/H2SO4which is known as Jones reagent. A solution of ferrous sulphate with hydrogen peroxide FeSO4/H2O2 is known as Fenton reagent. Hydrogen peroxide itself also works as an oxidizing agent. The Swarn oxidation and PCC are used to convert primary alcohol into acid.
Note:
Chromic acid and potassium permanganate convert primary, secondary alcohol, and ketone into a carboxylic acid. Chromic acid and potassium permanganate both first convert alcohol into aldehyde and then into a carboxylic acid. Both are strong oxidizing agents. Potassium permanganate works in an alkaline medium. Partial hydrolysis of the nitrile gives the amides and complete hydrolysis gives carboxylic acid.
