Question
Question: Propan-2-ol on reacting with \(C{{l}_{2}}\) produces: A. Trichloroethanol B. Trichloroacetone ...
Propan-2-ol on reacting with Cl2 produces:
A. Trichloroethanol
B. Trichloroacetone
C. Acetone
D. None of these
Solution
Think about which functional group among halogens and hydroxides is a better leaving group. Reason about what kind of reaction may occur as a consequence of the previous deduction.
Complete step by step solution:
This kind of reaction is known as the Haloform reaction, it forms compounds like chloroform, iodoform, etc.
Here, a displacement reaction substituting the OH− group with the Cl− group cannot occur as Cl− is a better leaving group than OH− and cannot be substituted. But chlorine is a very electronegative group and causes an elimination reaction. It oxidizes the secondary carbon by dehydrogenation and forms a ketone and HCl. The reaction occurs as follows:
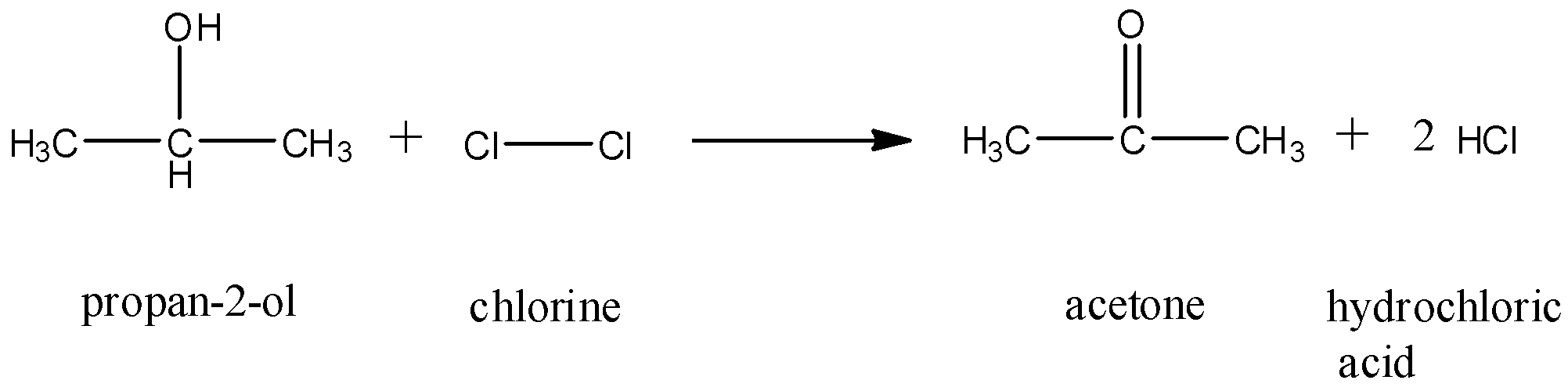
Acetone is the intermediate formed in this reaction.
Now, the excess of chlorine immediately reacts with the formed acetone to form a haloform compound. The chlorine replaces the hydrogen atoms on one of the carbon atoms and turns acetone into trichloroacetone. The reaction goes as follows:
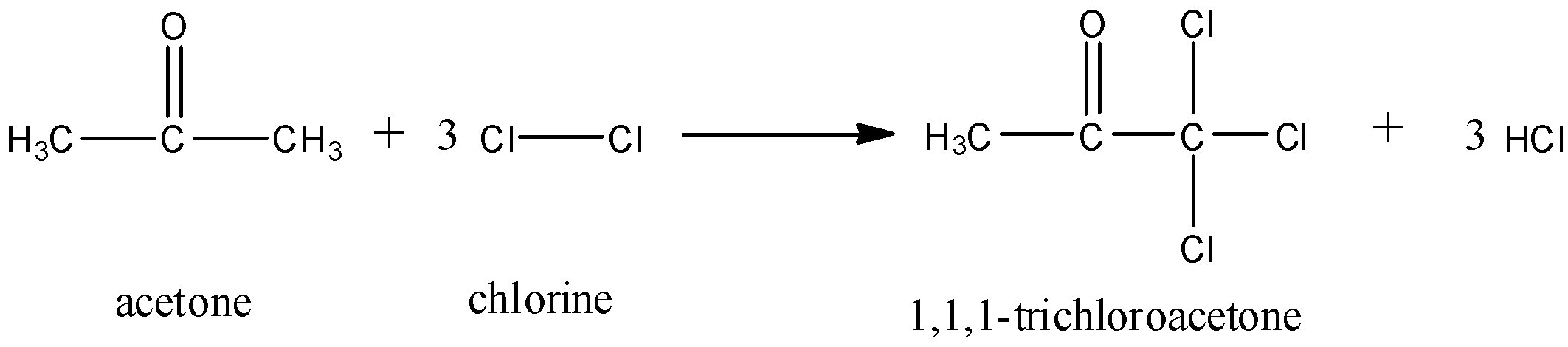
This reaction will go on till all the chlorine is depleted.
Hence, the answer is ‘B. Trichloroacetone’.
Note: The intermediate formed in this reaction is acetone. If chlorine is not available in excess then most of the product found will be acetone, but some of the product will still be trichloroacetone and some of the reactant will remain unreacted. This happens as some molecules may react and form products faster than others on the basis of pure chance. Do not get confused and mark your answer as ‘C. Acetone’ since the final product formed will always be trichloroacetone.
