Question
Question: Products obtained when cold \(HI\) reacts with isopropyl methyl ether at \(273K\) are: (A) Isoprop...
Products obtained when cold HI reacts with isopropyl methyl ether at 273K are:
(A) Isopropyl iodine and methyl alcohol
(B) Isopropyl alcohol and methyl iodine
(C) Isopropyl iodine and water
(D) Methyl iodine and water
Solution
HI reacts with isopropyl methyl ether by undergoing substitution reaction. Substitution reaction is a reaction in which one functional group of the compound gets substituted with another group. Substitution reaction is of two types i.e. nucleophilic substitution reaction and electrophilic substitution reaction.
Complete step by step answer:
When isopropyl methyl ether reacts with cold HI , it undergoes SN2 reaction. In SN2 reaction, the elimination of the leaving group and attack of the nucleophile occurs simultaneously.
In the reaction, isopropyl methyl ether (mixed ether) or reacting with cold HI yields methyl iodine (lower alkyl halide) and isopropyl alcohol (higher alcohol), as show in the reaction below:
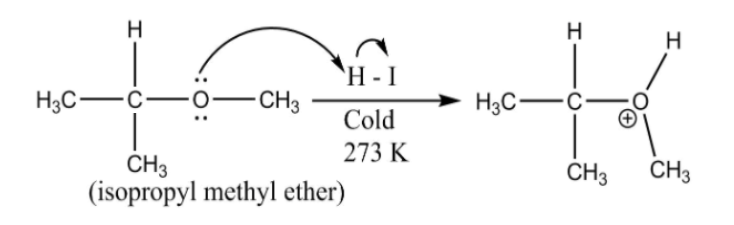
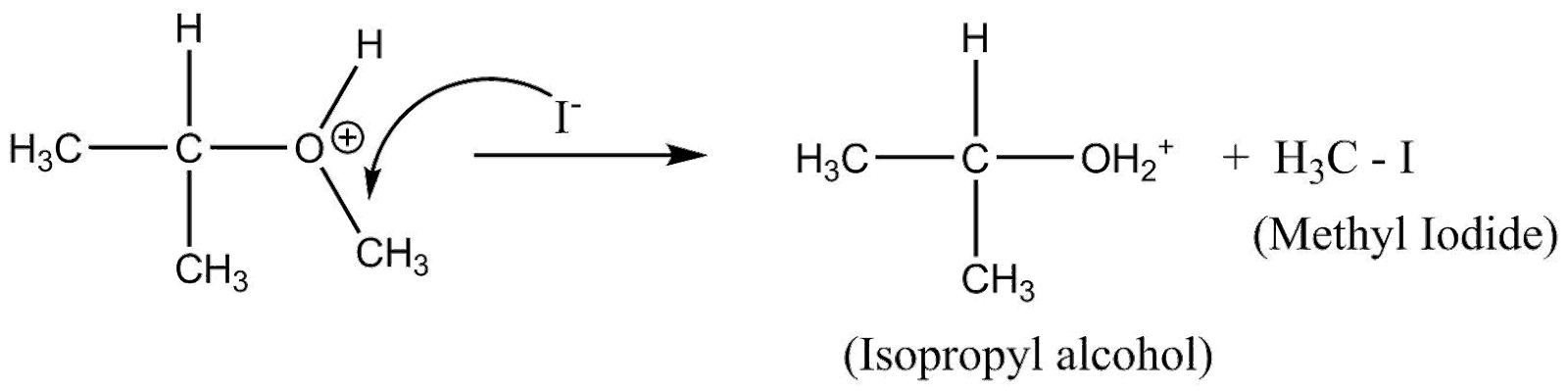
The (CH3)2−CH−O− is formed and H from H−I attaches to it. Whereas, the I from H−I attaches to −CH3 (leaving group)
Additional Information:
When isopropyl methyl ether is reacted with hot HI instead of cold HI , following reaction takes place at 373K
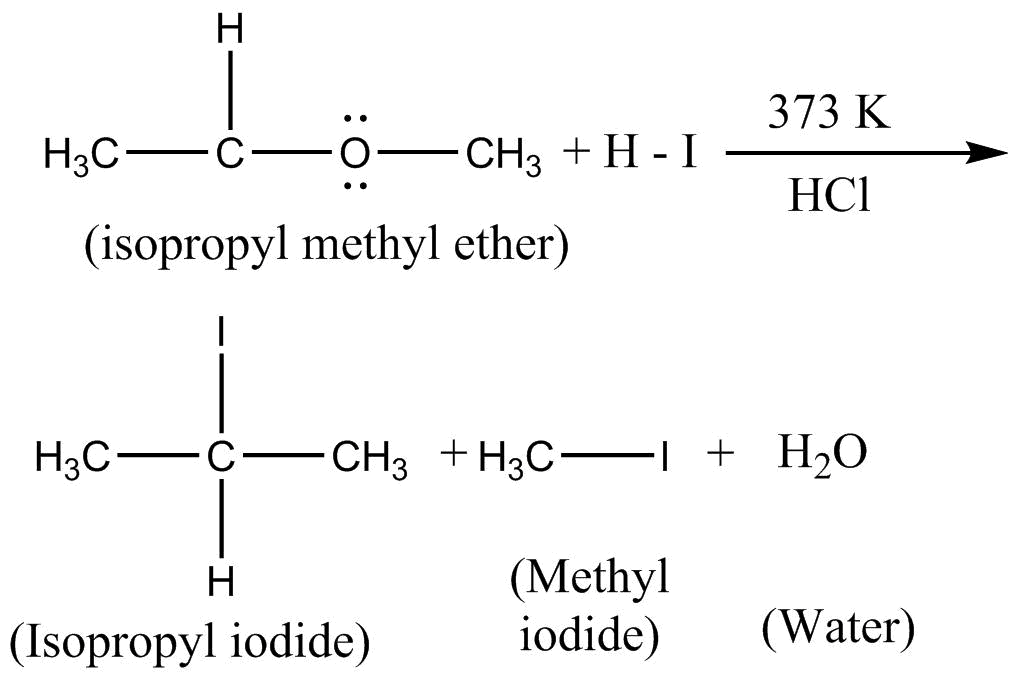
Hence, isopropyl iodine, methyl iodine and water are obtained upon the reaction of isopropyl methyl ether with hot conc HI .
So, the correct answer is Option B.
Note: SN2 reaction is a common reaction in organic chemistry. In this reaction one bond is broken and another bond is formed simultaneously. In this reaction, the nucleophile attacks from the back side of the substrate, to break the carbon-leaving group bond. It then forms a new carbon-nucleophile bond. For observing the maximum rate of SN2 reaction, the substrate must be least hindered. Therefore, the primary and methyl substrate show maximum rate of SN2 reaction, the secondary substrates have a lower rate of reaction and the tertiary substrate do not participate at all in the SN2 reaction because of large amount of steric hindrance in them.
