Question
Question: Products A and B formed in the given reactions are respectively: 
A.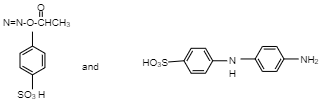
B.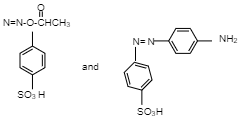
C.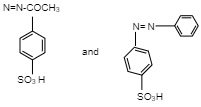
D.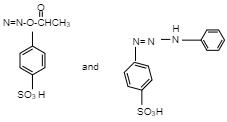
Solution
To find what products are formed when reaction is happened we need to be aware of the type of reaction resulting in the products. Production of diazonium salt and then reaction of another compound with these salts are types of reaction.
Complete step by step solution:
So if we see the given reaction two products are formed, that is two reactions happen. So if we see the first reaction. We will see the following result.
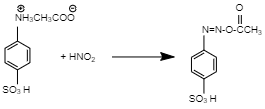
This reaction is known as diazotization as primary aromatic amine converted to diazonium salt. And now if we see the second reaction we will see the following result.
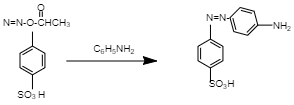
This reaction is known as azo coupling reactions because the diazonium salt which is product A reacted with another aromatic compound (aniline that is C6H5NH2 ) that produce azo compound.
So, Option B is correct.
Additional Information:
Aromatic compounds are chemical compounds that have conjugated planar rings along with delocalized pi-electrons in place of double and single bonds. Also they are known as arenes. Examples of aromatic compounds are toluene and benzene.
Diazotization reactions are when primary aromatic amine and nitrous acid is reacted to form a diazo compound. It is used to make various aromatic compounds by diazonium salts. Also to synthesize dyes.
Azo coupling is an organic reaction, when diazonium compound and another aromatic compound is reacted it results in formation of azo compound. Mostly the diazonium compounds are also aromatic. Mostly they are used as dyes known as azo dyes. Due to formation of coupling between compounds there are types of azo reaction, those are Azo C-coupling reactions and Azo N-coupling reactions. It is a common reaction which is used for preparation of derivatives of phenols and aromatic amines.
Note: When an aromatic compound is reacted with nitrous acid( HNO2 ) it results in formation of diazonium salts. And when diazonium salts are reacted with aniline they result in formation of azo compounds. Also to find products find the type of reaction happening.
