Question
Question: Product (X) is?  is?
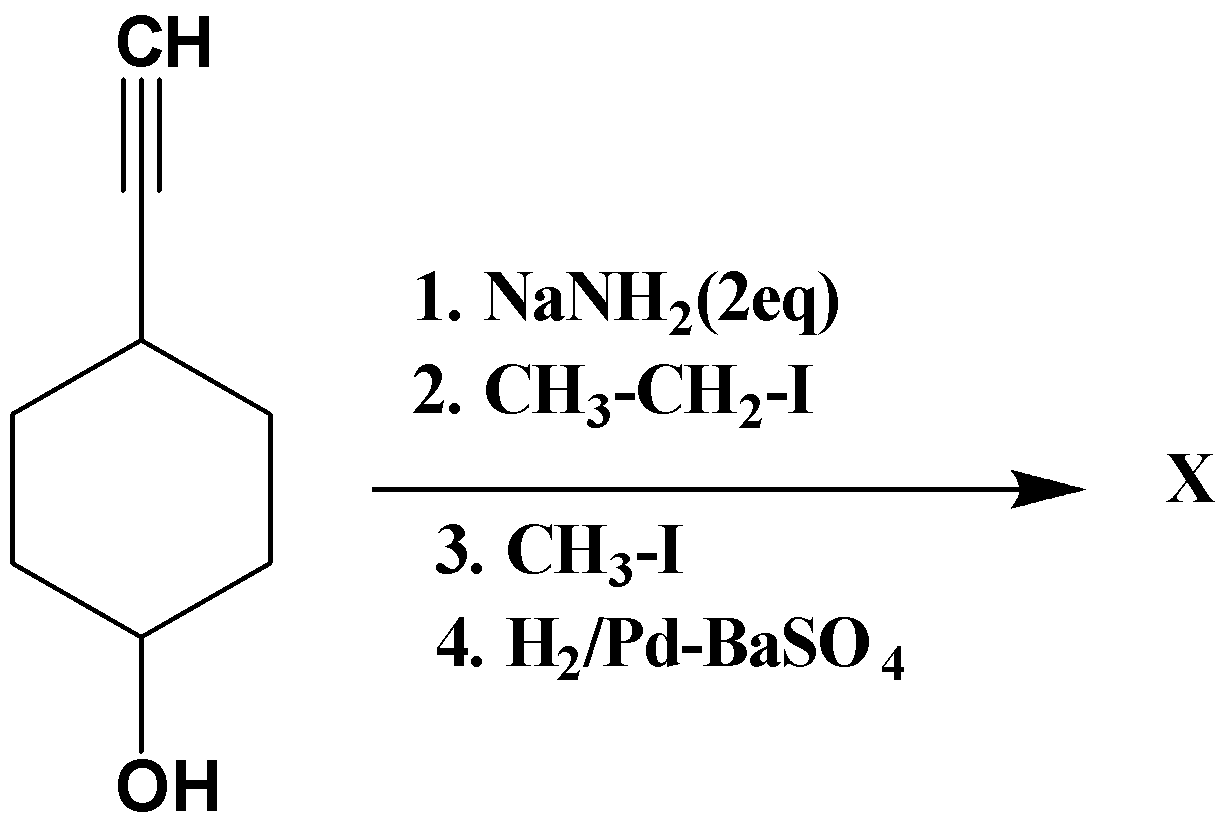
Solution
The basic function and nature of the different catalysts used during the different stages of reaction of the given molecule should be understood first. The reaction undergoes nucleophilic substitution with alkyl halide.
Complete step by step answer:
NaNH2 is a base, so it abstracts theH atom. Here within the structure two kinds of acidic hydrogens are present. One on the terminal alkyne and other one with the alcohol proton. 2 equivalents of base are used so both the protons will be removed by the base, the main concern is about which proton will be removed first. The proton attached to the terminal alkyne proton has a sp hybridization and because of this sp hybridization it is more acidic when compared to proton attached to alcohol, therefore it will be removed first and then the proton attached to alcohol will be removed. So in the first step the terminal alkyne H will be removed and further the alkyl halide CH3CH2I will undergo nucleophilic substitution reaction by which the leaving group is replaced by an electron rich compound. Then in the third step again CH3I will undergo nucleophilic substitution reaction with the alcohol and finally in the fourth step the hydrogenation of alkyne to alkene takes place.
Therefore, after the reaction the product X formed will be
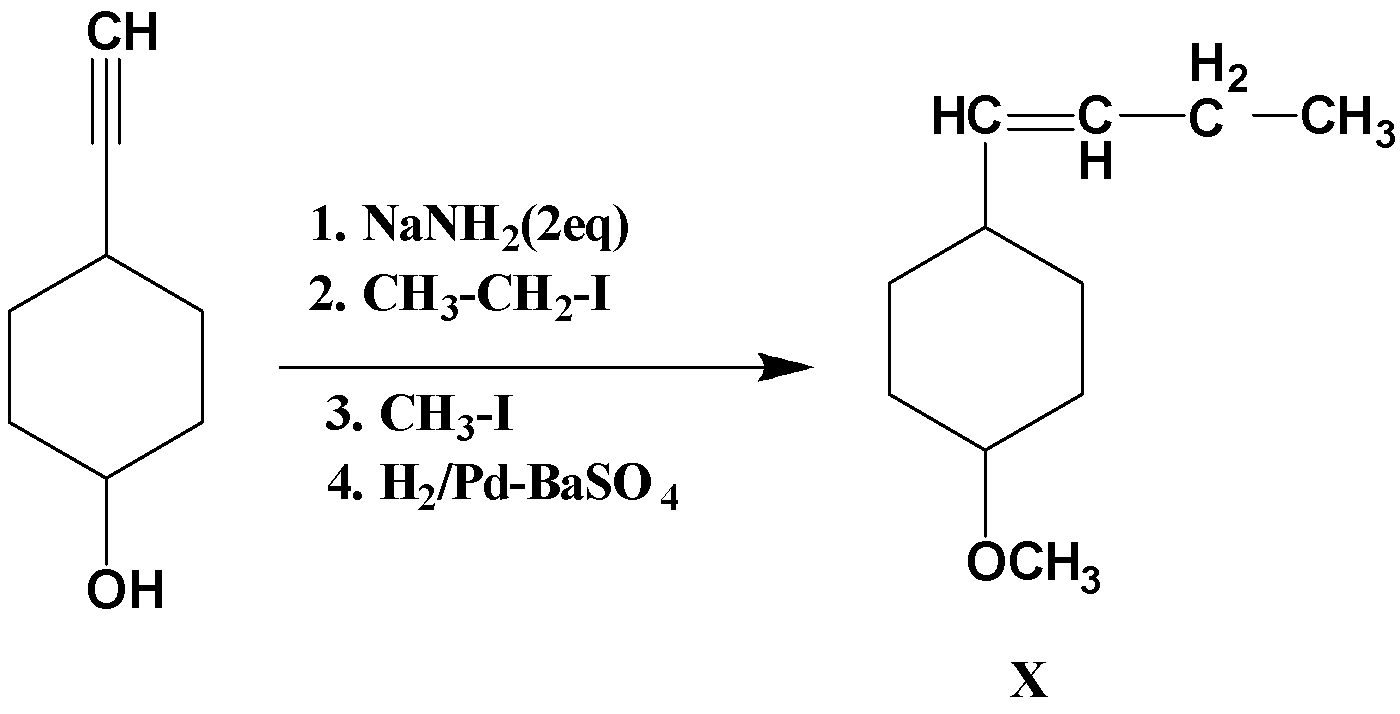
Note: The reactivity or strength in nucleophilic substitution reaction of nucleophile is known as nucleophilicity. Therefore a stronger nucleophile replaces a weaker nucleophile from its compound in a nucleophilic substitution reaction. Nucleophilicity is a kinetic term which relates to the rate at which the nucleophile attacks the substrates.
