Question
Question: Product of the reaction is: 
A.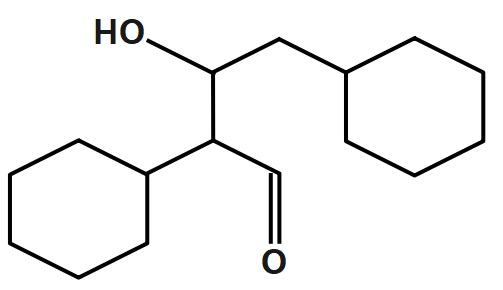
B.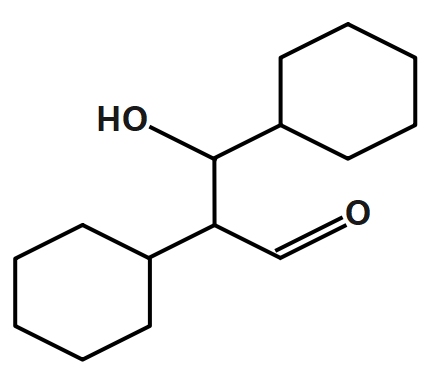
C.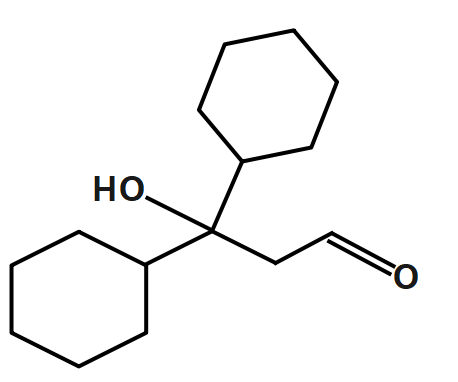
D.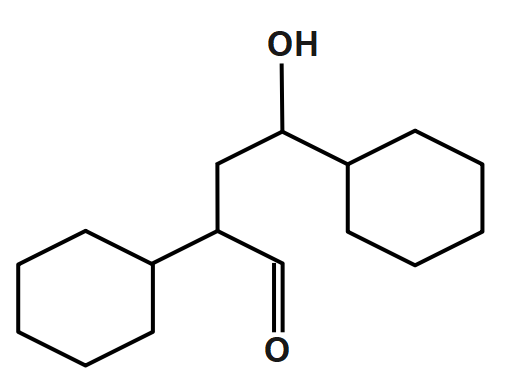
Solution
We know that here the given reagent sodium hydroxide and Water both give acid and salt reaction which is also commonly known as Neutralization reaction. Later, when an acid and base react with each other they form salt and water.
Complete answer:
As we know that the Sodium chloride and water are the products obtained on reaction of forms hydrochloric acid and sodium hydroxide base. The neutralization reaction can take place between strong acid, strong base or weak acid, strong base or strong acid, weak base, etc. Also, in a double decomposition reaction, mainly two ions are replaced with one another. Like cation is replaced by another cation and anion is replaced by another anion. In decomposition reactions, compounds decompose into two or more simpler compounds. Here the given compound firstly reacts with NaOH which provides OH− similarly the H2O compound gives respective compound and given below:
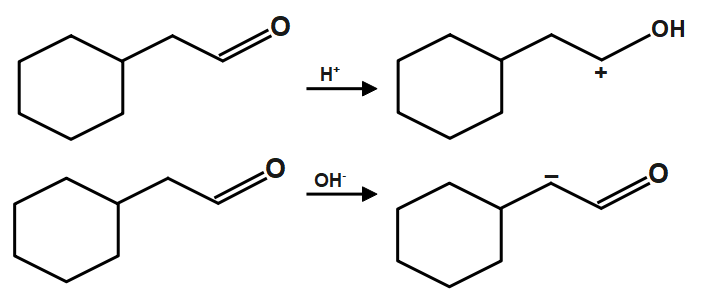
Now we just have to add both compound form from acid and base reaction of the original(given) compound. The given reagent NaOH,H2O are basically neutralizing reagents. When an acid and a base are placed together, they react to neutralize the acid and base properties, producing a salt. The H+ cation of the acid combines with the OH− anion of the base to form water. The compound formed by the cation of the base and the anion of the acid is called a salt.
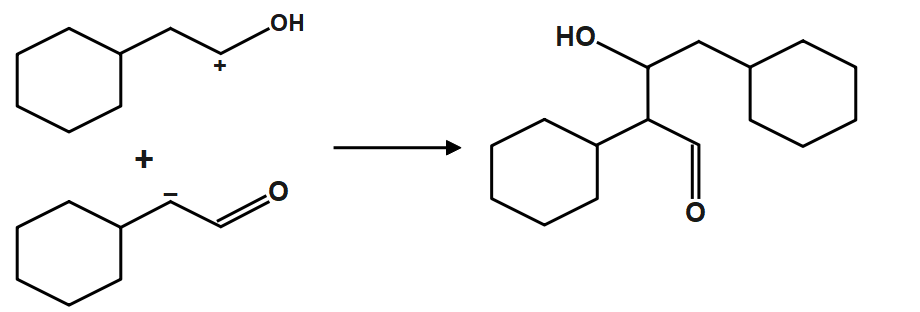
Therefore, the correct answer is option A.
Note:
Remember that the word salt is a general term which applies to the products of all such acid-base reactions. Acid–base reactions have importance in both biochemistry and industrial chemistry. The salts are classified into the various categories as Acidic acid, basic salt, double salts, complex salt, and simple salts.
