Question
Question: Product of the reaction is: 
A. 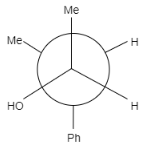
B. 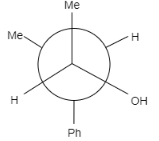
C. Both (A) and (B)
D. None of these
Solution
Grignard reagent is defined as a strong nucleophile that has a chemical formula RMgX. Here, R is the alkyl group and X is any halogen. Grignard reagent can behave as a carbonyl compound with electrophiles. It is used to form carbon- carbon bonds somewhat similar to organolithium.
Complete step-by-step answer:
Grignard reagent is prepared when magnesium is reacted with alkyl halides or aryl halides and form Grignard reagent. We can make these reagents with alkyl chlorides and alkyl bromides but not with alkyl fluorides. The solvent used for the synthesis of Grignard reagent is diethyl ether. The magnesium halide part of the reagent acts as a carbanion whereas the alkyl group acts as a cation.
Let us discuss the Grignard reaction mechanism-
The Grignard reagent which is nucleophilic attacks the electrophilic carbon of the carbonyl group. The Grignard reagent acts as a base therefore it attacks on the proton of the carbonyl compound. After that, the action of the Grignard reagent attacks on the carbon of the carbonyl group whereas the anion attacks on the oxygen atom.
In this question, the carbonyl compound is planar and shows sp2 geometry. When Grignard reagents Meδ+Mg−Br attacks on the given staggered compound, the attack can take place from any side. The probability of the attack of a nucleophile is equal for both the compounds.
Let us see the reaction of both the compounds-
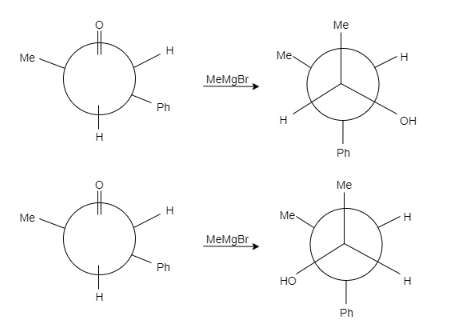
Therefore, formation of both the products is possible.
Hence the correct answer is C.
Note: It is to note that the major product formed is (b) because the hydroxyl group is present between the hydrogen group and phenyl group that means it is sterically less hindered whereas in option (a) the hydroxyl group is present in between the methyl and phenyl group, that means it will show more steric hindrance than (a) and minor product is formed.
