Question
Question: Product formed in the given reaction is: 
A.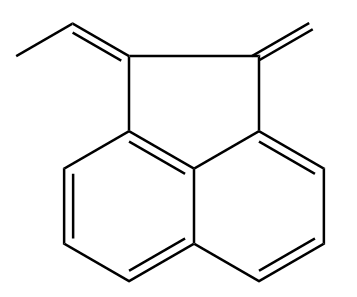
B.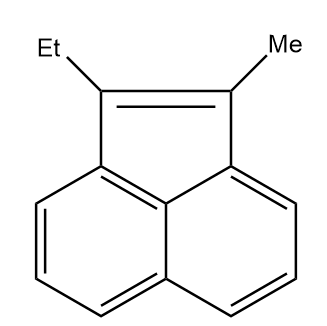
C.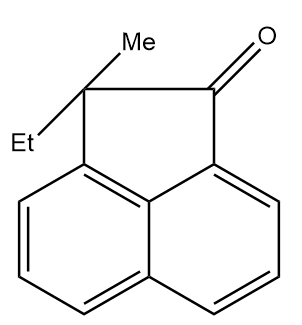
D.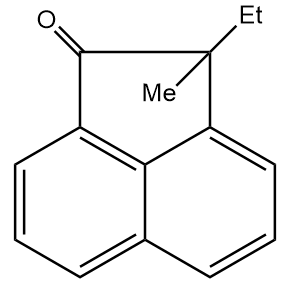
Solution
Pinacol pinacolone rearrangement is one of the most important organic reactions in which 1,2-rearrangement of a compound which consist of two hydroxyl groups, takes place and after the reaction, a compound is formed which consist of both a ketonic group as well as hydroxyl group and the reaction proceeds in acidic medium.
Complete answer:
The given reaction is an example of pinacol pinacolone rearrangement and the reaction mechanism is as follows:
Step-1: Since the reaction proceeds in acidic medium, the hydrogen ion will attack the hydroxyl group and protonation of the given compound will take place. The reaction proceeds as follows:
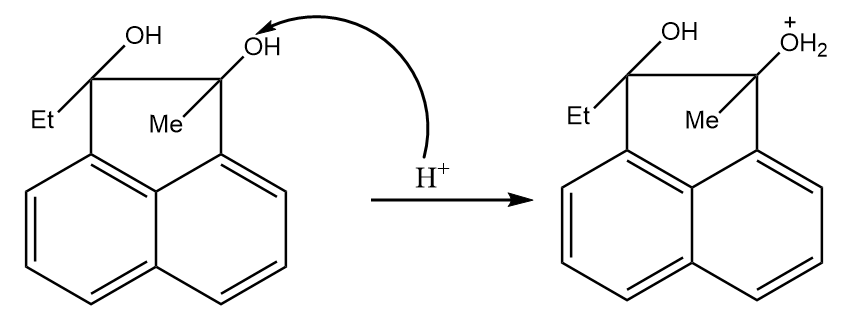
Step-2: Water is removed and a stable tertiary carbocation is formed as per following reaction:
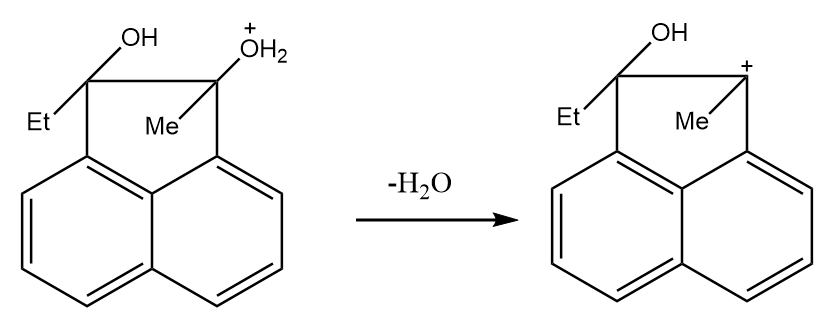
Step-3: The ethyl group migrates from the adjacent position to the positively charged carbon in the rearrangement of the compound. The reaction proceeds as follows:
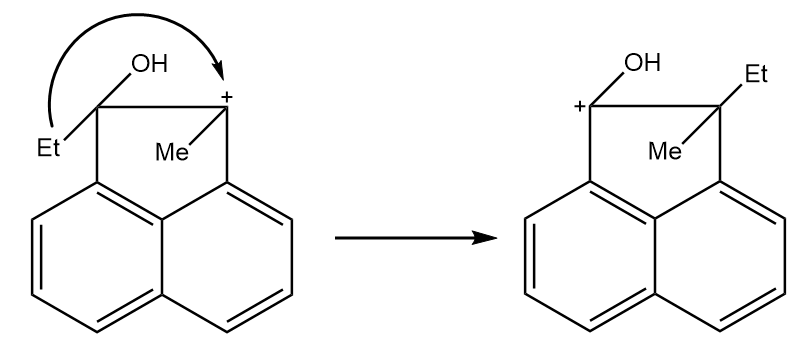
Step-4: Deprotonation of the doubly bonded oxygen atom takes place in order to yield the final product. The reaction proceeds as follows:
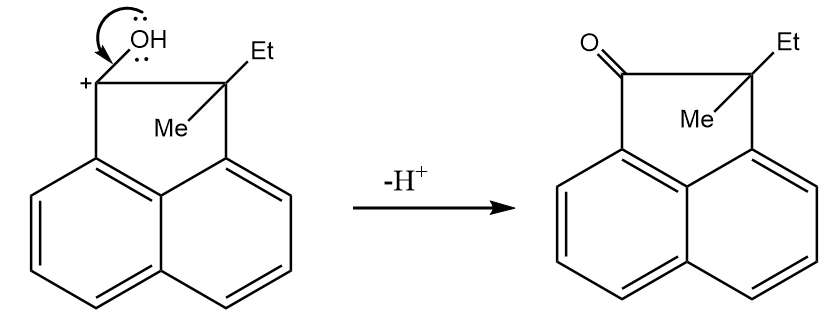
Hence, the final product formed is as follows:
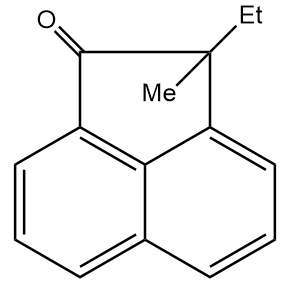
Thus, option (D) is the correct answer.
Note:
It is important to note that the attack of hydrogen ion will take place to the hydroxyl group which consist of a greater number of alpha hydrogens i.e., at the carbon atom which is bonded to methyl group will form more stable carbocation because the more the number of alpha hydrogen atoms, the greater will be the stability of the compound due to hyperconjugation.
