Question
Question: Product formed in the following reaction is/are : 
A. CH2OH and HCO2H
B. CH3OHand 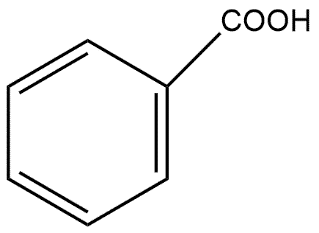
C. 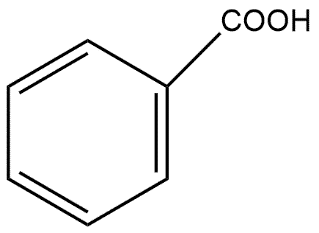 and
and 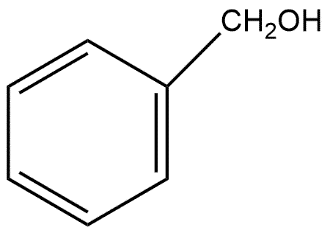
D. HCOOH and 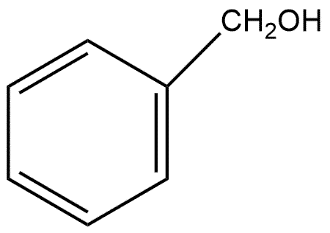
Solution
Reaction shown above is the example of crossed cannizzaro reaction. In this reaction aldehyde should have α hydrogens which undergo deprotonation due to strongly alkaline conditions of the reactions which form enolates.
Complete Solution :
- Aldehydes which have alpha hydrogen atoms undergo deprotonation due to the strongly alkaline conditions of the reaction which leads to the formation of enolates and by aldol reactions of these enolates beta-hydroxy aldehydes or ketones are obtained as a product. So we can say that this reaction produces only 50% of the required alcohol and carboxylic acid at ideal conditions. That’s why the crossed Cannizzaro reaction is more commonly used. A sacrificial aldehyde is combined with a more valuable chemical and formaldehyde is used as a reductant which oxidizes it to sodium formate. The required alcohol is obtained from the reduction of the other aldehyde. Since we can say that two different aldehydes can be completely converted into the required product and the yield of the valuable chemical is increased.
- Cannizzaro reaction is a redox process because in this process one aldehyde is oxidized to give a carboxylic acid whereas the other aldehyde undergoes reduction to yield the alcohol. Since both oxidation and reduction occurs in the hydride transfer that’s why the reaction is said to be a redox process.
So, the correct answer is “Option C”.
Note: The main advantage of crossed cannizzaro reaction is it improves the yield of the desired product. Both the aldehydes used are entirely converted to products and wastage of the valuable reactant chemicals is avoided. The atom economy of the process is also low.
