Question
Question: Product (B) is:  is:
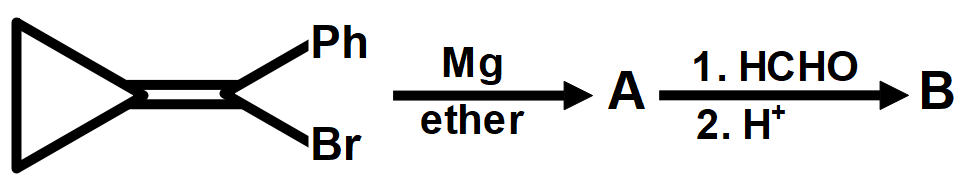
A.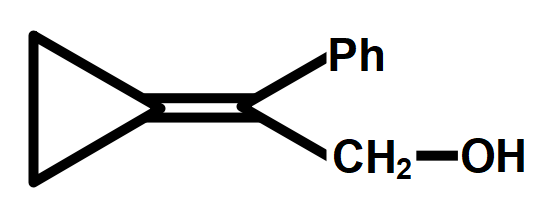
B.Ph−C≡C−CH2−CH2−CH2−OH
C.Ph−C≡C−CH2−CH2−OH
D.Ph−CH2−C≡C−CH2−CH2−OH
Solution
We know that the Carbonyl compounds are the organic compounds in which carbon-oxygen double bonds are present. In organic chemistry, one of the most essential functional groups is carbonyl carbon. Both the aldehyde and ketone groups have a carbonyl group, i.e. >C=O group and they give many chemical reactions like Nucleophilic addition reaction, reduction reaction, oxidation reaction, halogenations, and reaction with alkali.
Complete answer:
As we know that the Carbonyl compounds are mainly of two types- Aldehydes, and ketones. Aldehydes and ketones contain a carbonyl group which is referred to as a simple organic compound. These functional groups contain a carbon-oxygen double bond. In the carbonyl group, the carbon present lacks reactive groups such as Cl or OH which make these organic compounds simple. Aldehydes are the compounds in which hydrogen and carbon are attached to carbonyl groups whereas ketones are the compounds in which two carbons are attached to the carbonyl group.
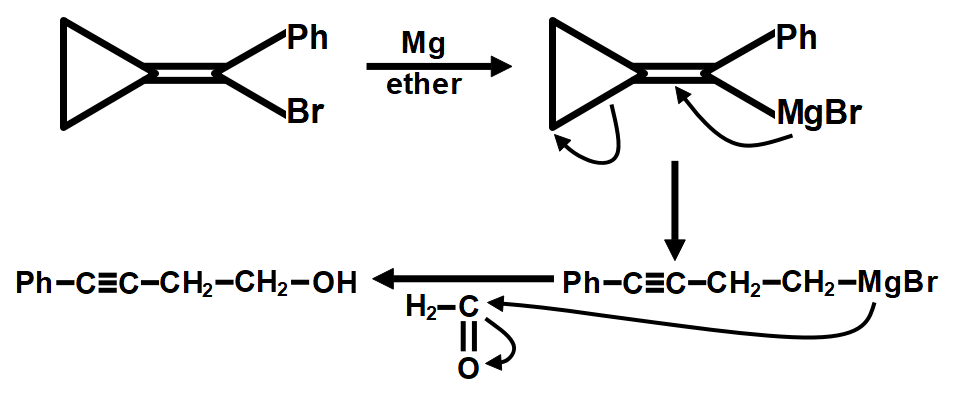
Therefore, the correct answer is option B.
Note:
Remember that the reaction of aldehydes and ketones with alkali’s is known as an aldol condensation reaction. Aldehydes and ketones show so many chemical reactions because they are very reactive. Also, Aldehydes can be prepared by several methods but one of the best ways for preparing the aldehydes includes by oxidation of primary alcohols. For the successful oxidation of primary alcohol, these mild oxidizing agents like DMP, PCC, and Swern are pretty much important.
