Question
Question: Product A in the below reaction is: \({C_6}{H_5}N{H_2}\,\, + \,\,CHC{l_3}\,\, + \,KOH\, \to \,A\, ...
Product A in the below reaction is:
C6H5NH2+CHCl3+KOH→A+KCl+H2O
A.

B.
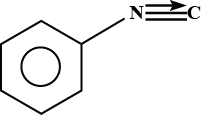
C.
D.
Solution
In this reaction. We have to identify the product A .In the reaction Aniline, chloroform and alcoholic potassium hydroxide on heating gives a foul smelling compound .
Complete step by step answer:
The given reaction is an example of isocyanide test , also known as Carbylamine reaction . When aliphatic or aromatic 1∘ amines reacts with chloroform in the presence of strong alkali then a foul smelling compound isocyanide is formed . The reaction which takes place is :
C6H5NH2+CHCl3+3KOH→C6H5NC+3KCl+3H2O
In this reaction phenyl isocyanide is formed which gives offensive smell .
Isocyanide test is used to distinguish primary amines from secondary and tertiary amines , as secondary and tertiary amines do not form foul smelling compounds when they react with chloroform and alcoholic potassium hydroxide .
The mechanism which takes place in the above reaction is described below .
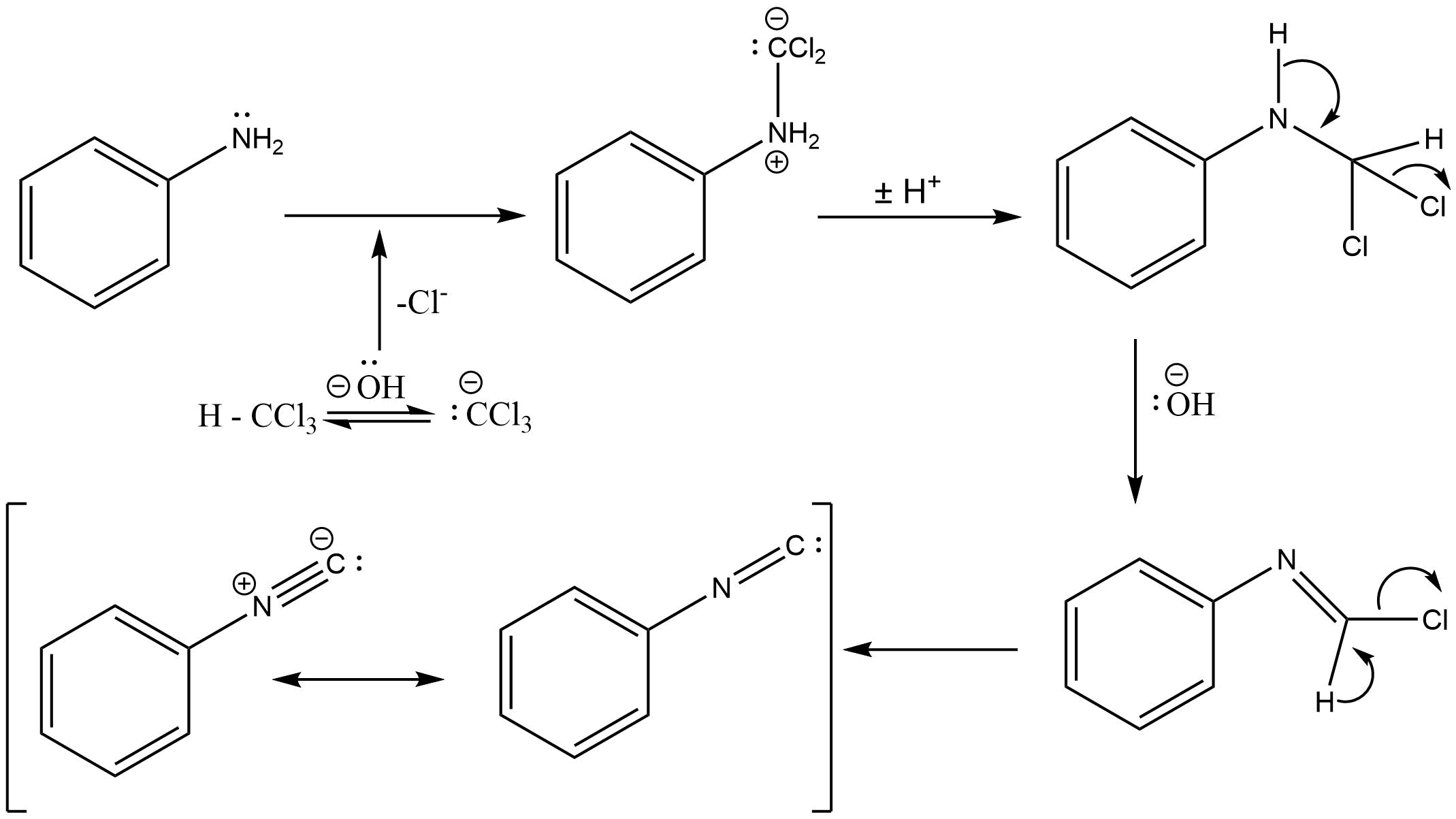
As you can see in the given figure , first aniline reacts with dichlorocarbene ( which is formed from dehydrohalogenation of chloroform ) , then the intermediate which is formed is very reactive . The attack of electrophilic dichlorocarbene takes place on the nucleophilic centre of the primary amine , finally hydrochloric acid is eliminated from the compound and isonitrile is formed .
Hence , the product formed is phenyl isocyanide .
So , option B is correct .
Note: We can use the carbylamine reaction to synthesize isocyanides using primary amines and reacting them with chloroform and a base . We can also use this test to check for the presence of a primary amine in a given substrate .
