Question
Question: Problem 2.11 : 0.01m aqueous formic acid solution freezes at -0.021 °C. Calculate its degree of diss...
Problem 2.11 : 0.01m aqueous formic acid solution freezes at -0.021 °C. Calculate its degree of dissociation. Kf = 1.86 K kg mol−1 ΔTf = i Kfm
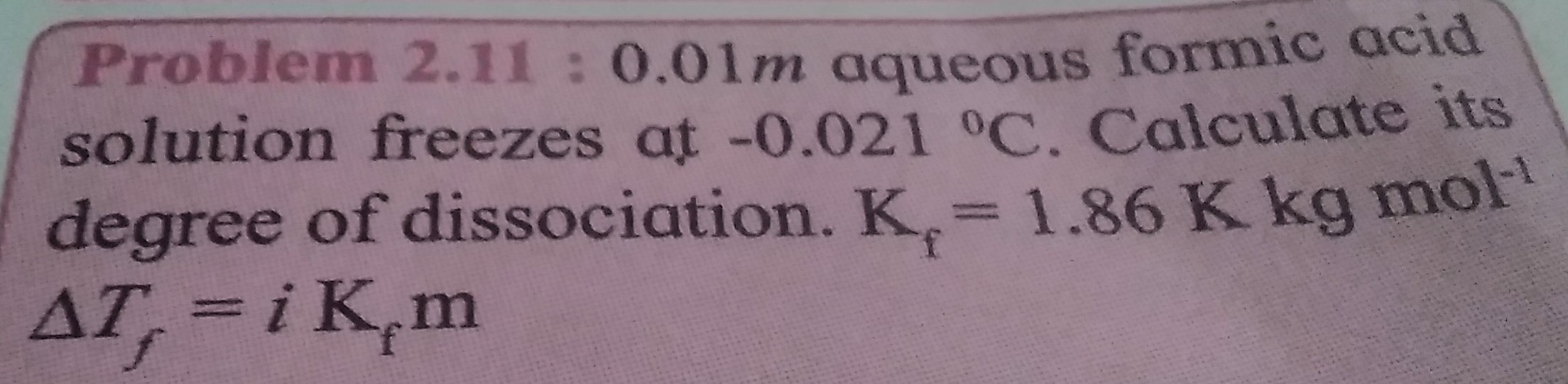
Answer
0.129
Explanation
Solution
- Calculate the freezing point depression ΔTf=Tf∘−Tf=0−(−0.021)=0.021 °C = 0.021 K.
- Use the formula ΔTf=iKfm to find the van't Hoff factor i. Substitute the known values: 0.021=i×1.86×0.01.
- Solve for i: i=1.86×0.010.021=0.01860.021=3135.
- For the dissociation of formic acid (HCOOH⇌HCOO−+H+), the van't Hoff factor is related to the degree of dissociation (α) by i=1+α.
- Substitute the value of i and solve for α: 3135=1+α⟹α=3135−1=314.
- Convert the fraction to a decimal and round to an appropriate number of significant figures: α=314≈0.129.
