Question
Question: Primary alkyl halide \({C_4}{H_9}Br\) (a) reacted with alcoholic \(KOH\) to give compound (b). Compo...
Primary alkyl halide C4H9Br (a) reacted with alcoholic KOH to give compound (b). Compound (b) is reacted with HBr to give (c) which is an isomer of (a). When (a) is reacted with sodium metal it gives compound (d), C8H18 which is different from the compound formed when n-butyl bromide is reacted with sodium. Give the formula of (a) and write the equations for all the reactions.
Solution
Hint: We have learned that there are two types of primary alkyl halides with the same molecular formula C4H9Br that is n-butyl bromide and isobutyl bromide. So the first step is to find out which type of primary halide is used.
Complete answer:
In the problem it is given that when (a) is reacted with sodium metal it gives compound (d) , C8H18 which is different from the compound formed when n-butyl bromide is reacted with sodium hence now we now that the compound (a) is isobutyl bromide and compound (d ) is 2,5-methylhexane. So n-octane then 2,3-methyl hexane is obtained by the following reaction .
2CH3CH2CH2CH2Br+2Na→CH3CH2CH2CH2CH2CH2CH2CH3
2CH3CH(CH3)CH2Br+2Na→CH3CH(CH3)CH2CH2CH(CH3)CH3 ,
This reaction is also called the Wurtz reaction. Now take a look at this reaction which is when (a) reacted with alcohol KOH to give compound (b) so we know that (a) is isobutyl bromide.
CH3CH(CH3)CH2Br→CH3C(CH3)=CH2
So here we see that the compound (b) is 2-methyl-1-propene.
Compound (b) is reacted with HBr to give (c) which is an isomer of (a) ,now see this reaction and find out compound (c), it is important to remember that here product formation will be according to Markownikoff’s rule as the rule states that Hydrogen is added to the carbon which has the more number of hydrogen and the halide part is added to the carbon which has the least number of carbon. So the reaction is as follows:
CH3CH(CH3)=CH2→CH3−CBr(CH3)CH3
Here the product is tert- butyl bromide which is an isomer of isobutyl bromide. Structural formula of isobutyl is shown in figure: 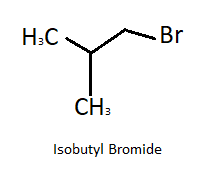
Note: In this sequence of reactions first we have identified the compound (a) which is iso butylbromide, then we write each reaction as it was given in the problem in order to get all the compounds.
