Question
Question: Primary alcohols are obtained by the reaction of Grignard reagent with: A. \({\text{C}}{{\text{H}}...
Primary alcohols are obtained by the reaction of Grignard reagent with:
A. CH3COCH3
B. HCOOH
C. HCHO
D .CH3CHO
Solution
We should know what Grignard reagent is and how it works. The general formula of Grignard reagent is RMgX. It gives R−. R− attacks on electrophilic centres. We will determine the electrophilic centre. We should also know what primary alcohol is. From all the products we will determine the primary alcohol.
Complete Solution :
The alkyl magnesium halide reagent having general formula RMgX is known as a Grignard reagent. This reagent is used for the generation of alkyl nucleophiles. The alkyl nucleophile attack on carbonyl carbon generates negative change on carbonyl oxygen which gets protonated to give alcohol.
The reaction of prop −2− on CH3COCH3 with Grignard reagent is as follows:
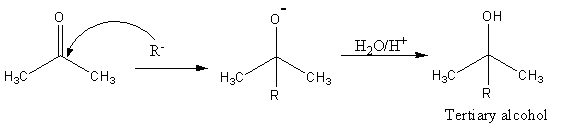
The reaction prop −2− on with Grignard reagent gives tertiary alcohol.
The reaction of formic acid with Grignard reagent is as follows:
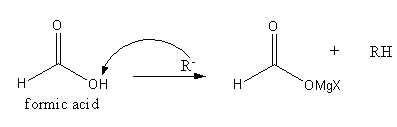
The hydrogen attached with oxygen is very acidic so the alkyl group generated from Grignard reagent abstracts this acidic hydrogen forming RH and magnesium salt of carboxylic acid as products.
The reaction of formaldehyde HCHO with Grignard reagent is as follows:
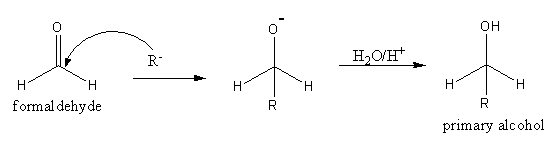
The reaction formaldehyde with Grignard reagent gives primary alcohol.
The reaction of acetaldehyde CH3CHO with Grignard reagent is as follows:
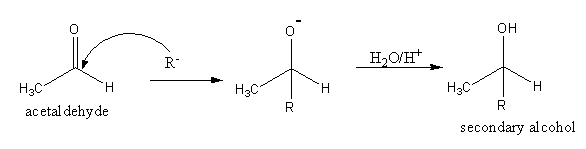
The reaction acetaldehyde with Grignard reagent gives secondary alcohol.
So, primary alcohols are obtained by the reaction of the Grignard reagent with formaldehyde HCHO.
Therefore, option (C) HCHO, is correct.
Note: Carbonyl carbon is the most electrophilic centre. Carbonyl is of two types; aldehydes and ketones. Only the formaldehyde gives primary alcohol. Remaining all aldehydes give secondary alcohol. Ketones do not give primary alcohol. Ketone gives secondary and tertiary alcohol. Prop on is a ketone and acetaldehyde is an aldehyde rather than formaldehyde, so both do not give primary alcohol. Acids react differently with the Grignard reagent. Acid gives alkanes.
