Question
Question: Preparation of benzene from phenol is: Reduction Oxidation Addition Dehydrogenation...
Preparation of benzene from phenol is:
Reduction
Oxidation
Addition
Dehydrogenation
Solution
We know that benzene is a cyclic hydrocarbon and has a chemical formula C6H6. Each carbon atom in benzene is arranged in a six-membered ring and is attached to only one hydrogen atom
Complete step by step answer:
Based molecular orbital theory for benzene structure, benzene ring involves the formation of three delocalized π orbitals across all six carbon atoms, while the valence bond theory describes two stable resonance structures for the benzene ring.
Benzene is a volatile and flammable compound. It is highly toxic and smells like gasoline. It is carcinogenic.
We can find benzene in crude oil that is unrefined petroleum and it is also obtained as a natural product by refining oil.
Benzene is immiscible in water and does not form a homogeneous solution with water but in organic solvents, it is soluble.
Benzene is produced through volcanoes and forest fires naturally. There are several laboratories and industrial techniques for the preparation of benzene.
In commercial scale, benzene is mainly obtained from coal tar.
We can prepare Benzene from phenols through their reduction. In this process, phenol vapours are passed over heated zinc dust. Zinc dust reduces them to produce benzene. We can write the chemical reaction as,
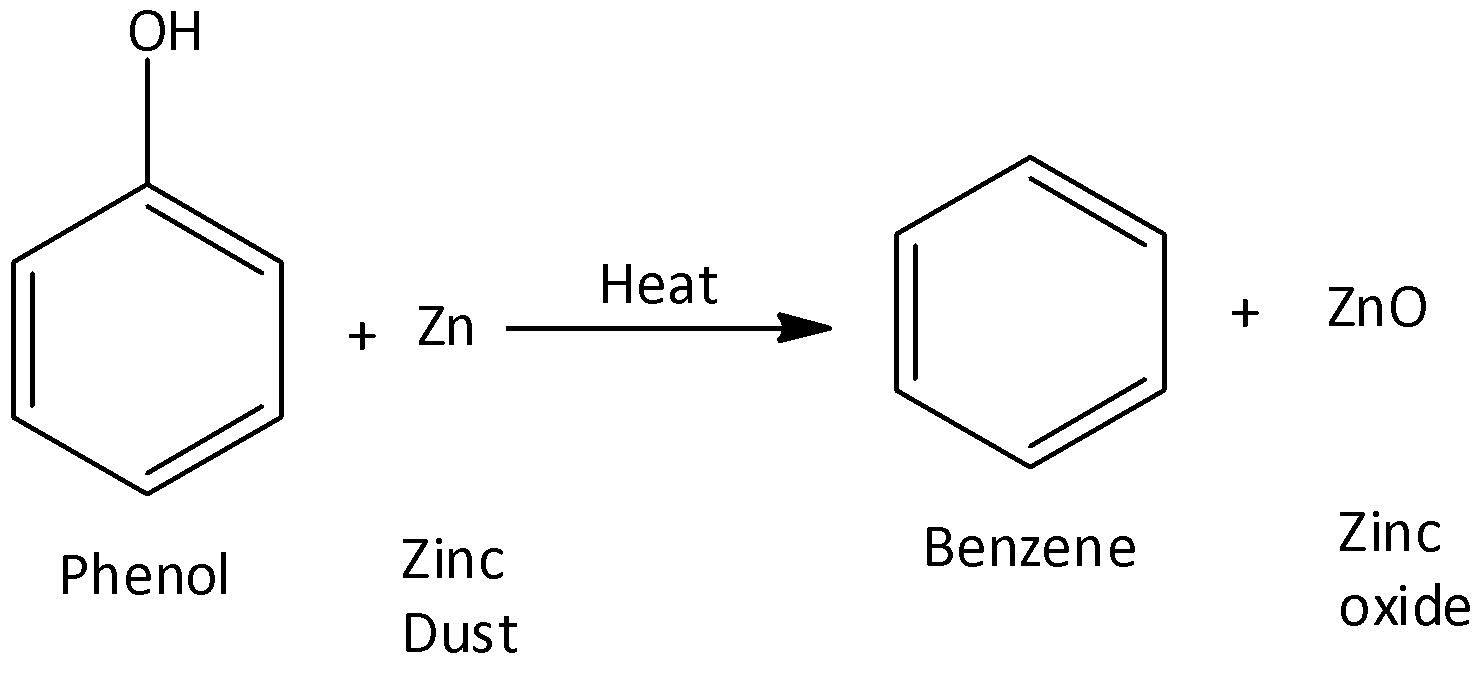
So, the correct answer is Option A .
Note:
We also know the some of the uses of benzene and which are listed below,
Widely used in the production of pesticides, detergents, and resins.
For domestic purposes, we use benzene in glue, adhesive, cleaning products, tobacco etc.
We can also use benzene to prepare aniline and phenol.
It is used in degreasing metals.
Used in the manufacture of chemicals like ethylbenzene, cumene, nitrobenzene, cyclohexane etc.
