Question
Question: Predict the products formed when cyclohexane carbaldehyde reacts with the following reagents: (i)-...
Predict the products formed when cyclohexane carbaldehyde reacts with the following reagents:
(i)- PhMgBr and then H3O+
(ii)- Tollen’s reagent
(iii)- Semicarbazide and weak acid
(iv)- Excess ethanol and acid
(v)- Zinc amalgam and dilute hydrochloric acid
Solution
Cyclohexane carbaldehyde means there is a ring of six carbon atoms and on one carbon atom of the ring, an aldehyde group is present. When the aldehyde reacts with tollen’s reagent it reduces it.
Complete step-by-step answer:
The structure of cyclohexane carbaldehyde is given below:
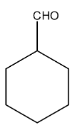
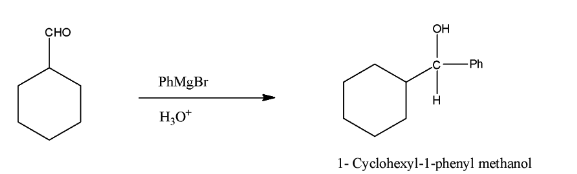
(i)- When this compound reacts with PhMgBr and then H3O+, it will form alcohol because PhMgBr is a Grignard reagent and then it is hydrolyzed, the product will be 1- Cyclohexyl-1-phenyl methanol. The reaction is given below:
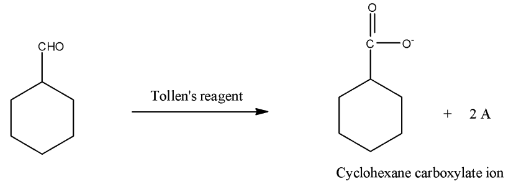
(ii)- With tollen’s reagent, it will get oxidized to carboxylate ion because it can reduce the Tollen’s reagent and will give a silver mirror test. The reaction is given below:
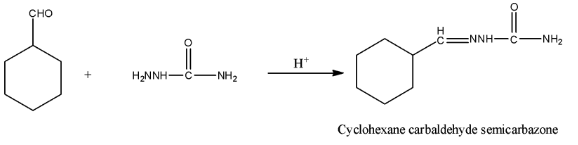
(iii)- With Semicarbazide and a weak acid, there will be a reduction of the water molecule and there will be the formation of Cyclohexane carbaldehyde semicarbazone. The reaction is given below:
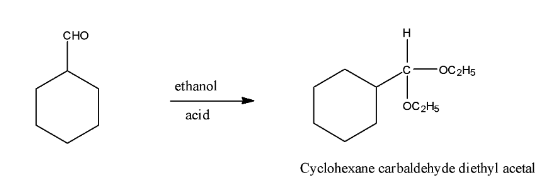
(iv)- With Excess ethanol and acid it will form acetals because the double bond between the carbon and oxygen will break and there will be the addition of −OC2H5. The product will be Cyclohexane carbaldehyde diethyl acetal. The reaction is given below:
(v)- With zinc amalgam and dilute hydrochloric acid the aldehyde group of the compound will be reduced to a methyl group. The product will be 1-Methyl cyclohexane. The reaction is given below:
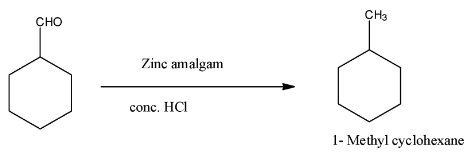
Note: When any aldehyde is reduced with zinc amalgam and concentrated hydrochloric acid to form alkanes, the reaction is known as Clemmensen’s reduction, and even the ketones are also reduced with this reagent.
