Question
Question: Predict the product formed in the following reaction. 
A. 3-methyl-2-butanone
B. 3, 3-dimethylmalonic acid
C. propane
D. 3, 3-dimethyl-2, 4-pentanedione
Solution
When an organic compound contains a ketonic group along with a carboxylic group, then it is called as a keto acid. The naming of keto acids can also be done as oxo alkanoic acids.
Complete step by step answer: We have been given a compound which is subjected to heat, and we have to find the product obtained. The compound contains a keto acid, with a 4 carbon chain along with 2 methyl branches. So, the name of this compound is 2,2-dimethyl-3-oxobutanoic acid. This keto acid when subjected to heat converts into di carboxylic acid, which is 3, 3-dimethylmalonic acid . The reaction is as,
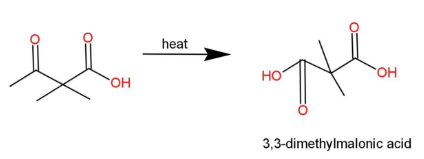
The reaction forms di carboxylic acid, as by heating of this keto acid, catalytic oxidation occurs, which creates an intermediate of carbanion, that has a hydroxyl group attached to form a stable 3, 3-dimethylmalonic acid.
Additional information: Keto acids are of various types, like alpha keto acids, that contain a keto group adjacent to the carboxyl group. Beta-keto acids that have a ketone group placed at the second carbon from the carboxyl group.
Hence, the reaction gives 3, 3-dimethylmalonic acid, so, option B is correct.
Note: Keto acids on heating have a removal of carboxyl ions. But here it is converted to 3, 3-dimethylmalonic acid, due to the compound being beta- keto acid heated at high temperature range produces an enol intermediate, hence the product here.
