Question
Question: Predict the basicity of the final product (having sulphur) obtained when \({\text{S}}{{\text{F}}_4}\...
Predict the basicity of the final product (having sulphur) obtained when SF4 undergoes hydrolysis.
Solution
The basicity is defined as the strength of a base to accept a proton. The basicity of an acid is determined as the number of protons donated by the acid to a base. Here we have to predict the basicity of the product obtained after hydrolysis of SF4.
Complete answer:
Hydrolysis of a compound is the dissolution of the compound in water, so the bonds in the compound break, and a new compound forms.
The hydrolysis of sulphur tetrafluoride SF4is as follows:
SF4+3H2O→H2SO3 + 4HF
Sulphur tetrafluoride gives acid, sulphurous acid, and hydrogen fluoride as the final products of hydrolysis.
The structure of the final product sulphurous acid (having sulphur) is shown as follows:
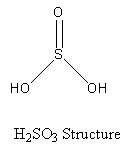
Basicity of an acid is the number of protons donated by the acid to a base.
It can be seen in the structure of the sulphurous acid, that the sulphurous acid has two hydroxyl groups. So, the sulphurous acid can donate two protons to a base, so the basicity of the sulphurous acid is two.
Therefore, the basicity of the final product (sulphurous acid) obtained when SF4 undergoes hydrolysis is 2.
Note: The central atom of the main product of hydrolysis has the same oxidation state as the oxidation state of the compound which is hydrolyzed. The acids of sulphur have one sulphur-oxygen double bond and remaining oxygen atoms and hydrogen atoms in hydroxyl form and then the left hydrogen atoms bound directly with sulphur atom. The hydrogen bound in the form of a hydroxyl group can only cause acidity.
