Question
Question: Potassium dichromate in alkaline solution with \[30\% \] \[{H_2}{O_2}\] produces \[{K_3}Cr{O_8}\]. H...
Potassium dichromate in alkaline solution with 30% H2O2 produces K3CrO8. How many peroxide linkages are found in the structure of K3CrO8?
Solution
To solve this question, first write the complete balanced equation for the given reaction. Here, the reaction is a redox reaction. Now, to find the number of linkages, use the formula for calculating the number of peroxide linkages. Before that, we will need the oxidation number of elements as well to be used in the formula.
Complete answer:
When potassium dichromate reacts with hydrogen peroxide, the following redox reaction is carried out:
K2Cr2O7+7H2O2+4KOH(alk)→2K3CrO8+9H2O
In the reaction, Hydrogen peroxide acts as an oxidizing agent and gets reduced. Its oxidation number changes to −2 from −1.
The number of peroxide linkages formed here is 4 and can be verified by drawing the structure of K3CrO8.
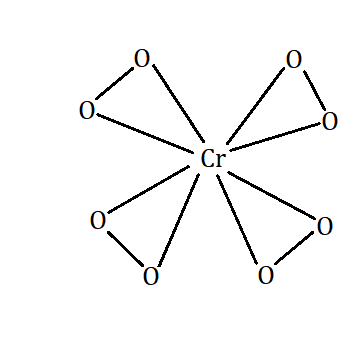
The number of peroxide linkages in a compound can be calculated by using the formula = (Theoretical oxidation number) − (Maximum oxidation number) /2.
The theoretical oxidation state of Cr− in K3CrO8 will be =16−3=13
The maximum number of linkages in the structure [CrO8]3− are =5
The number of peroxide linkages according to the formula can be calculated as =2(13−5)=4
Hence, the number of peroxide linkage is 4.
Note:
Whenever hydrogen peroxide is added to dichromate or any other Cr(VI) compound, then a blue colour is obtained. This blue colour is due to the formation of CrO(O2). However, the blue colour fades away and the compound decomposes readily into Cr3+ and O2(g) in aqueous solution. The peroxo compound can be extracted into oxygenated organic solvent like diethyl ether, ethyl acetate or 1−pentanol etc. where it remains stable.
