Question
Question: Potassium crystallize in body-centered cubic lattice with the edge length an equal to\({\text{5}}{\t...
Potassium crystallize in body-centered cubic lattice with the edge length an equal to5.2Ao, then what is the distance (inAo) between nearest neighbours?
A. 2.25
B.3.0
C.2.5
D. none of the above
Solution
In case of body-centered cubic unit cell, the one-fourth of diagonal length represents the radius. The diagonal edge length of the body-centered cubic unit cell is a3. We will determine the radius of the atom present in BCC first then the distance (inAo) between nearest neighbours. In BCC two atoms touch each other.
Formula used: r=4a3
Complete step-by-step solution: The formula which is used to determine the atomic radius of a body-centered cubic unit cell is given as follows:
r=4a3
Where,
ris the atomic radius.
a is the edge length of the unit cell.
Substitute 5.2Ao for the edge length of the unit cell.
r=45.2Ao×3
r=2.25Ao
So, the atomic radius of the body-centered cubic unit cell of the potassium atom is2.25Ao.
The neighbour atoms in BCC are shown as follows:
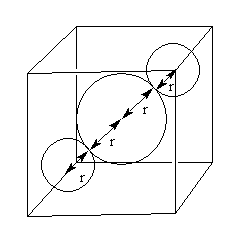
The distance between two neighbour atoms is 2r so,
=2×2.25Ao
=4.5Ao
So, the distance (inAo) between nearest neighbours is 4.5Ao.
Therefore, option (D) none of the above, is correct.
Additional information: Different types of unit cell have different edge length and radius. In face-centered cubic lattice, the diagonal edge length is a2 so, the radius is the one-fourth of the edge length a2 . The formula to determine the radius of face-centered cubic lattice is as follows:
r=4a2
In case of simple cubic unit cell, the edge length is taken as a so, the formula which relates the edge length with atomic radius is a = 2r.
Note: The atomic radius is one-fourth of the edge length for the BCC unit cell. The edge length depends upon the type of unit cell, so the relationship between edge length and atomic radius also depends upon the type of unit cell. In the different unit cells, the arrangement of atoms is different. So, the edge length is different.
