Question
Question: Polythene is prepared by : (A) Isomerization (B) Polymerisation (C) Hydrogenation (D) All of...
Polythene is prepared by :
(A) Isomerization
(B) Polymerisation
(C) Hydrogenation
(D) All of the above
Solution
As the name suggests , polythene is a polymer of ethene monomer and it is prepared by polymerising the ethene at higher temperatures.
Complete step by step solution:
Before deciding by which process polythene is prepared , let us first understand what each of these reactions means.
Isomerization : This refers to the type of process in which a compound is transformed into an isomer with a different chemical structure and same chemical formula .
Polymerisation: It is a chemical reaction in which smaller units called monomers combine to form a large molecule called polymer. The combining units i.e. monomers can be the same or different molecules.
Hydrogenation: It is simply the addition of hydrogen to any compound . The alkynes are converted to alkenes by the process called hydrogenation.
Now , we have known what each of the processes mean .
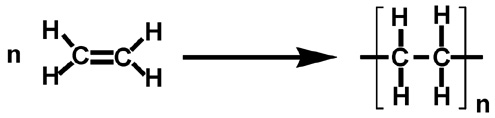
Ethene is taken as a monomeric unit here and a large number of these monomeric units are combined with each other at elevated temperatures. The process is carried out at a temperature range of 350∘C to 570∘C and at very high pressures of about 1000 to 2000 atm. Ethene is a stable molecule which in addition to catalysts participate in the polymerisation process.
Polythene as the name suggests is the polymer of ethene . Now , we know that polythene is prepared by the polymerisation of ethene. It is a homo-polymer i.e. it consists of the same type of monomers which is ethene.
Hence, the correct answer is B .
Note:
Poly means “many”, when two or more simpler units combine to form a more complex meaning by some means of chemical and physical process , is called polymerisation. The polymers are of two types i.e addition polymers and condensation polymers.
