Question
Question: Polarity in a molecule and hence the dipole moment depends primarily on the shape of the molecule an...
Polarity in a molecule and hence the dipole moment depends primarily on the shape of the molecule and electronegativity of the constituent atoms. Which of the molecules has the highest dipole moment?
A. CO2 $$$$
B. HI
C. H2O
D. SO2
Solution
dipole moment of a bond A-B is charge accumulated on either A and B without sign multiplied with the length of bond A-B.
Complete step by step solution: Let's analyze the options one by one, CO2 is a triatomic molecule where there is a slight difference between the electronegativity of C and O that is oxygen is more electronegative than carbon but the structure of CO2 is linear. One carbon atom joins the two oxygen atoms with a double bond with each of them and makes a 180 degree angle overall. Therefore the dipole moment thus observed along the direction of carbon to oxygen atoms on both the sides cancel each other making the net dipole moment zero.
HI is a diatomic molecule where there is high electronegativity difference between hydrogen and iodine as iodine is a group 17 member group of highest electronegativity elements. But the electronegativity in the group decreases as one moves down the group hence iodine is the least electronegative element and its electronegativity is less than oxygen.
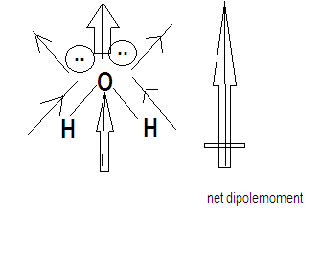
H2O is a triatomic molecule with an angular structure. Oxygen is more electronegative than hydrogen. Both the lone pair and the dipole moment of both the O-H bonds contribute in one direction which is why H2O has a maximum dipole moment .
SO2 is also a triatomic molecule but sulphur and oxygen does not have a high electronegativity difference. Its dipole moment is reduced by difference in the direction of dipole moment contributed by the double bond and lone pair.
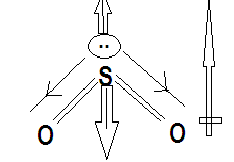
Hence the correct option is option C.
Note: For diatomic heteroatomic molecules, the bond moment is equal to the dipole moment which is zero as there is no shifting of bond pair towards any atom.
