Question
Question: Point out the incorrect statement of the about resonance? A) Resonance structures should have equa...
Point out the incorrect statement of the about resonance?
A) Resonance structures should have equal energy.
B) In resonance structures, the constituted atoms must be in the same positions.
C) In resonance structures, there should not be the same number of electron pairs.
D) Resonance structures should differ only in the location of electrons around the constituent atoms.
Solution
We know that the relocation of electrons from the multiple bonds or a lone pair of electrons from an atom to another atom or an adjacent single covalent bond are called resonance. The variation of structures in the molecule or ion is called resonating, canonical, or contributing structures.
Complete step by step answer:
-The phenomenon of the subsistence of a molecule in many structures due to the delocalization of electrons is defined as resonance.
-The resonance structures are similar in energy, bonding, and nonbonding pairs of electrons only the distribution of electrons is different.
Let us draw different resonating structures of carbonate ions.
The resonating structure of carbonate ion is given as,
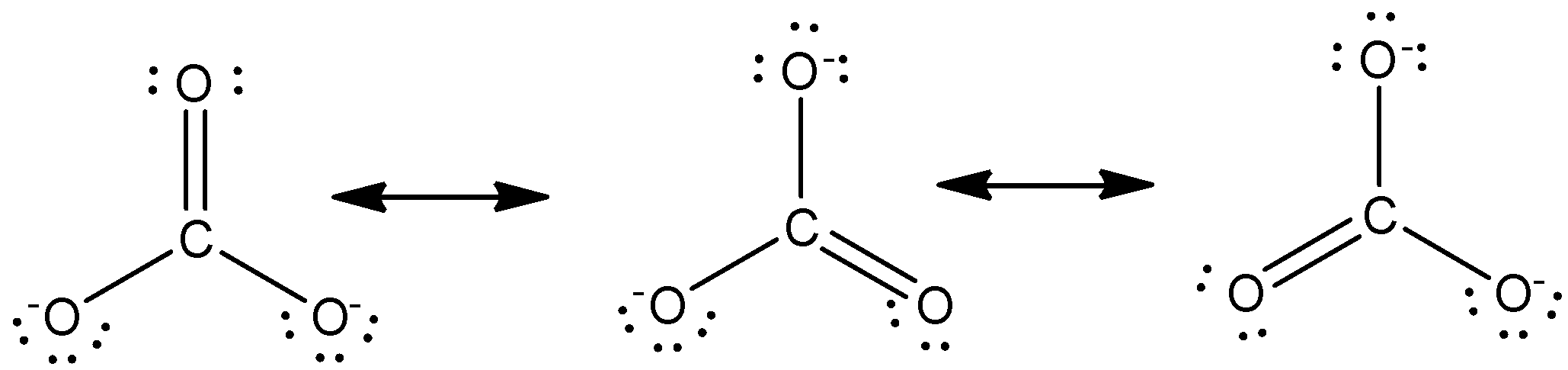
In the higher than structures, the center atom is secure to a few gas atoms. As these atoms are with chemicals identical, so any of those atoms will carry a charge or is bonded to the carbon atoms by a covalent bond. These reverberating structures take issue solely within the distribution of electrons however otherwise the same. Therefore in every resonance structure, each oxygen atom can be bonded by a double bond whereas the remaining two oxygen atoms will possess a negative charge.
We know that the resonance structures should have equivalent energy. In resonance structures, all the atoms must be in the same location. In resonance structures, there should not be an equal number of electron pairs. Hence the statement A, B and D are correct.
We know that the resonance structure should have the same number of electron pairs. Thus the incorrect statement about the resonance structure is C.
Therefore, the option C is correct.
Note:
We must remember that the resonance may be a way to portray the combination of several contributing structures into a hybrid resonance in valence bond theory in certain molecules or ions. The possibility of making mistakes is that the negative charge persists on oxygen atoms but the atoms are identical in any of these atoms can carry a negative charge..
