Question
Question: Point out the correct decreasing order of \({\text{p}}{{\text{K}}_{\text{b}}}\)values of the followi...
Point out the correct decreasing order of pKbvalues of the following amines:
C2H5NH2 ,C6H5NHCH3, (C2H5)2NHand C6H5NH2
A. (C2H5)2NH>C2H5NH2 > C6H5NHCH3 > C6H5NH2
B. (C2H5)2NH > C6H5NHCH3 > C6H5NH2>C2H5NH2
C. C6H5NH2 > C6H5NHCH3>C2H5NH2>(C2H5)2NH
D. C2H5NH2>(C2H5)2NH>C6H5NHCH3>C6H5NH2
Solution
The basicity is defined as the tendency of a molecule to donate the electrons or hydroxide ion. AS the availability of the electrons increases, the basicity of the molecule increases. The lower the pKbvalues higher will be the basicity.
Complete Step by step answer: In the amines, the nitrogen atom has a lone pair of electrons that can be donated so, the amines are basic.
Aliphatic amines are more basic than the aromatic amine.
Because in aromatic amine the phenyl ring works as an electron-withdrawing group which decreases the electron density on the nitrogen atom.
So, C2H5NH2 and (C2H5)2NH are more basic than C6H5NHCH3 and C6H5NH2.
In the case of aliphatic amines C2H5NH2 and (C2H5)2NH, the basicity is decided on the basis of steric hindrance and +I effect.
In the amine, the −NH2 group is an electron-withdrawing group so, the −NH2 group has +I effect. Thus, it withdraws the electron density from the alkyl chain so, as the number of alkyl groups increases the basicity should be increased.
So, according to the +I effect, the basicity order should be,
(C2H5)2NH > C2H5NH2
The +I effect in C2H5NH2 and (C2H5)2NH, is as follows:
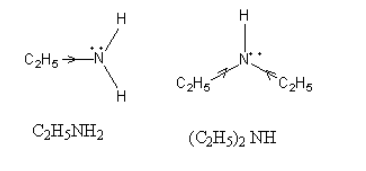
As the steric hindrance in amine increases the hydrogen ion of water which takes an electron from amine cannot approach the amine so, the basicity decreases.
So, according to the steric hindrance, the basicity order should be,
C2H5NH2>(C2H5)2NH
The order of basicity which comes by the combined effect of +I and steric hindrance is as follows:
(C2H5)2NH > C2H5NH2
In the case of aromatic amineC6H5NHCH3 and C6H5NH2., the basicity is decided on the basis of +I effect of methyl group.
The −I effect in C6H5NHCH3 and C6H5NH2, is as follows:
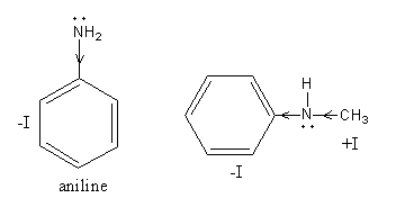
In C6H5NHCH3, one methyl group is present instead of hydrogen and methyl group shows +Ieffect which increases the electron density hence basicity.
So, the decreasing order of basicity is as follows:
(C2H5)2NH>C2H5NH2 > C6H5NHCH3 > C6H5NH2
Kb shows the strength of a base so, as the value of Kb increases the basicity increases and as thepKb value increases the basicity decreases.
So, a strong base will have low pKb.
The decreasing order of pKbvalues is as follows:
C6H5NH2 > C6H5NHCH3>C2H5NH2>(C2H5)2NH
Therefore, option (C) is correct.
C6H5NH2 > C6H5NHCH3>C2H5NH2>(C2H5)2NH
Note: Basicity is directly proportional to +I effect and inversely proportional to the −I and the steric hindrance. Basicity is also directly proportional to the number of electron donating groups. As the number of electron donating groups increases the basicity increases. The basicity of aniline is minimum among all four due to −I of the ring.
