Question
Question: Phthalic anhydride reacts with resorcinol in the presence of concentrated \({{H}_{2}}S{{O}_{4}}\) to...
Phthalic anhydride reacts with resorcinol in the presence of concentrated H2SO4 to give:
(A) Phenolphthalein
(B) alizarin
(C) coumarin
(D) fluorescein
Solution
Phthalic acid is a dicarboxylic acid. It reacts with a dehydrating agent i.e. sulphuric acid. Phthalic acid loses water on heating and acts as a dehydrating agent. The reaction of phthalic anhydride with resorcinol results in the formation of organic dye. This dye has a wide application as a fluorescent material in the laser application.
Complete Solution :
We have been provided that phthalic acid with resorcinol in the presence of concentrated sulphuric acid,
Phthalic acid is an aromatic dicarboxylic acid, with formula C6H4(CO2H)2 .It is an isomer of isophthalic acid and terephthalic acid. Resorcinol is an organic compound with the formula C6H4(OH)2. It is one of three isomeric benzenediols.
- When these two compounds react together in presence of concentrated sulphuric acid,
Sulphuric acid acts as a dehydrating agent because a substance that absorbs moisture from its surroundings is called a dehydrating agent. Sulphuric acid readily protonated H2O leading to the formation of hydronium ions.
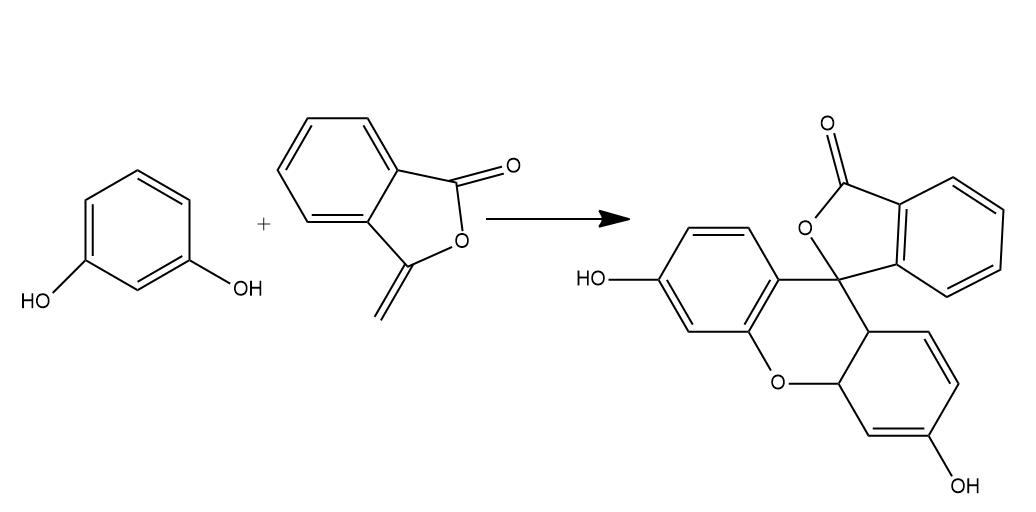
- Phthalic acid reacts with resorcinol in the presence of concentrated H2SO4 to give fluorescein. Phthalic acid gets dehydrated on treatment with concentrated H2SO4 to form phthalic anhydride. Phthalic anhydride further reacts with resorcinol to form fluorescein.
- So, we can say that Phthalic anhydride reacts with resorcinol in the presence of concentrated H2SO4 to give fluorescein.
So, the correct answer is “Option D”.
Note: Note that Adolf Von Baeyer first synthesized fluorescein in the laboratory. He prepared it from the phallic anhydride and resorcinol in presence of zinc chloride through the Friedel-Crafts reaction. There is one more method for synthesis using methane sulphonic acid . This gives a high yield.
Sulphuric acid is not used as a drying agent H2S because it reacts with it to form sulphur.
H2SO4+H2S→2H2O+SO2+S
