Question
Question: Phenylmethanol can be prepared by reducing the benzaldehyde with: A. \(C{H_3}Br\) B. \(Zn\) and ...
Phenylmethanol can be prepared by reducing the benzaldehyde with:
A. CH3Br
B. Zn and HCl
C. CH3Br and Na
D. CH3I and Mg
Solution
Phenylmethanol or Benzyl alcohol is an aromatic alcohol with formula, C6H5CH2OH. The structure of phenylmethanol is shown below. It is generally found in fruits and teas. It is a colourless liquid and has pleasant mild odour. It is generally used as an organic solvent.
Attribution: Leyo, Public domain, via Wikimedia Commons
Benzaldehyde is an aromatic aldehyde and can be converted into diphenylmethanol by reduction reaction.
Complete step by step answer:
Phenylmethanol can be prepared from benzaldehyde by treating it with zinc in presence of HCl.This is categorised as a reduction reaction as hydrogen is added to the molecule during this reaction.
Given below is the chemical reaction for the same.
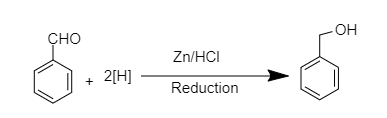
So, the correct answer is Option B.
Note: Benzaldehyde (C6H5CHO) – It is a compound containing formyl group. This is a colourless liquid. Benzaldehyde can be extracted from other natural sources which can be used to flavour cakes and baked goods.
There are a variety of methods used for reducing carbonyl compounds (aldehydes and ketones). One of the methods used is use of Grignard Reagent.
When Grignard reagents react with aldehydes and ketones, it forms additional products which decompose with dil. HCl or dil. H2SO4 to give primary, secondary and tertiary alcohols. Formaldehyde gives primary alcohol whereas all other aldehydes give secondary alcohols and ketones furnish tertiary alcohols.
Here, students might be confused about the fact that the reduction of benzaldehyde can also be carried out using Grignard reagent, CH3I and Mg to produce benzyl alcohol. But, remember Phenyl methanol is an aromatic primary alcohol hence, it cannot be produced by reduction of benzaldehyde using Grignard reagent. The reaction between benzaldehyde with Grignard reagent will yield secondary alcohol.
