Question
Question: Phenol reacts with \(PC{l_5}\) , the main product is: A. 
B. 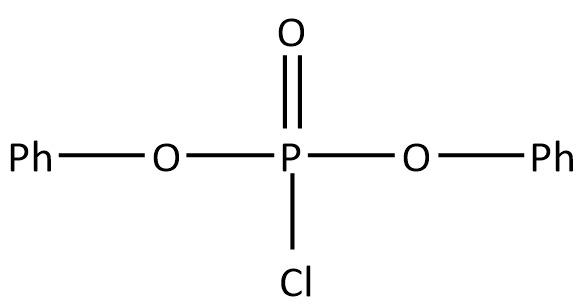
C. 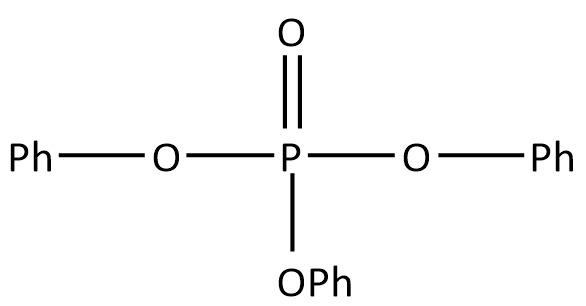
D. None of these
Solution
We know that in general, phosphorus pentachloride can be used for preparing alkyl chlorides from alcohol.
Complete step by step answer:
Functional groups impart specific characteristics as it is evident from the name itself. Based on the functional groups, we can have different categories of compounds. Let’s have a look at some of them.
Alkanes are simple hydrocarbons made up of only carbon and hydrogen atoms with a general formula of CnH2n+2 where n is an integer.
Alkyl groups: These are derived from alkanes by removing one hydrogen atom and are present as substituents. We generally represent them with R− which has been derived from the alkane RH .
Haloalkanes: These are also derived from alkanes by substituting hydrogen atom(s) with halogen(s). Halogens come under functional groups and are usually represented by X . So, we can use RX for haloalkanes. As it is evident that there is one alkyl group connected to halogen which gives rise to another name for these compounds to be alkyl halides.
Alcohols: In this category, the functional group is represented by OH− . Here also, hydrogen atom(s) in alkanes are replaced and we get ROH .
As we can see that all of these are inter-related this led us to devise methods for preparing one from another. It has been found that generally, alcohols reacting with PCl5 give alkyl chlorides. We can write the involved reaction as follows:
R−OH+PCl5→R−Cl+POCl3+HCl
However, when phenol in which the alcohol group is attached to the benzene ring, is treated with PCl5, it is quite different because in phenol, the C−O bond is much stronger. So, when phenol reacts with PCl5, following reaction takes place:
PhO−H+PCl5→PhO−PCl4+HCl
We can draw the structure for as follows:
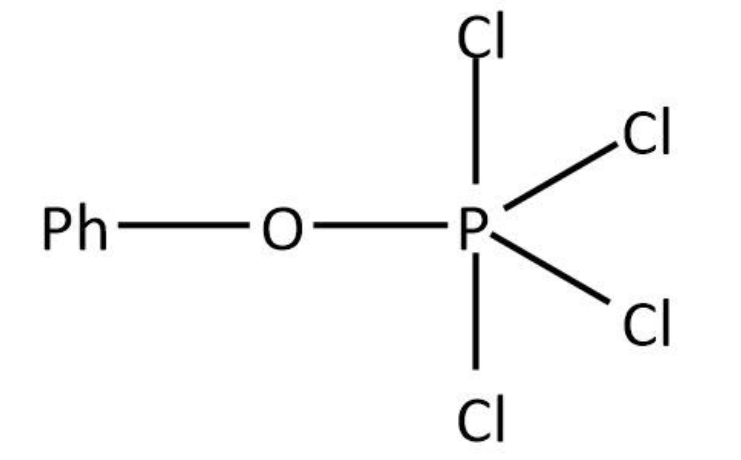
Therefore, the correct option is option (D).
Note:
We have to keep in mind that even though the functional group is the same, alkyl alcohols and aryl alcohols react differently.
