Question
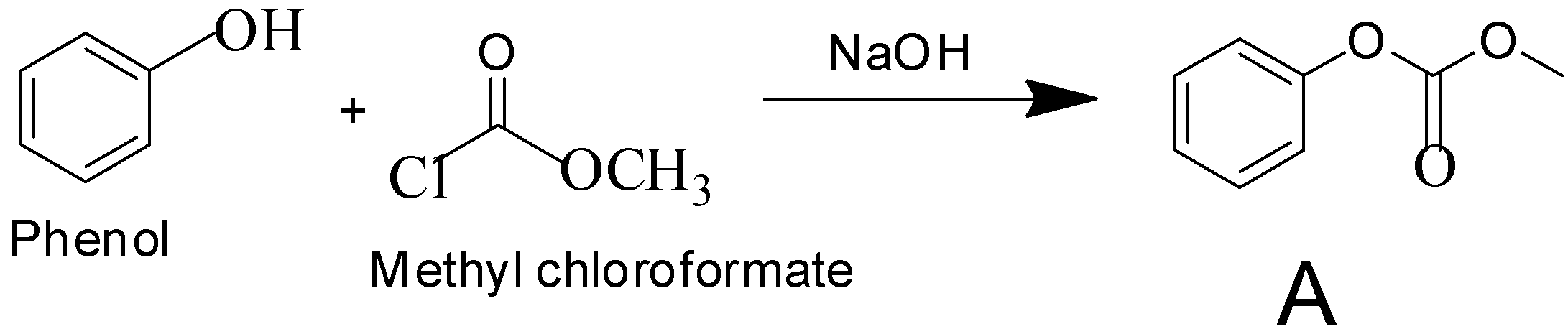
Question: Phenol reacts with methyl chloroformate in the presence of \(NaOH\) to form product A. A reacts with...
Phenol reacts with methyl chloroformate in the presence of NaOH to form product A. A reacts with Br2 to form product B. A and B are respectively:
A.
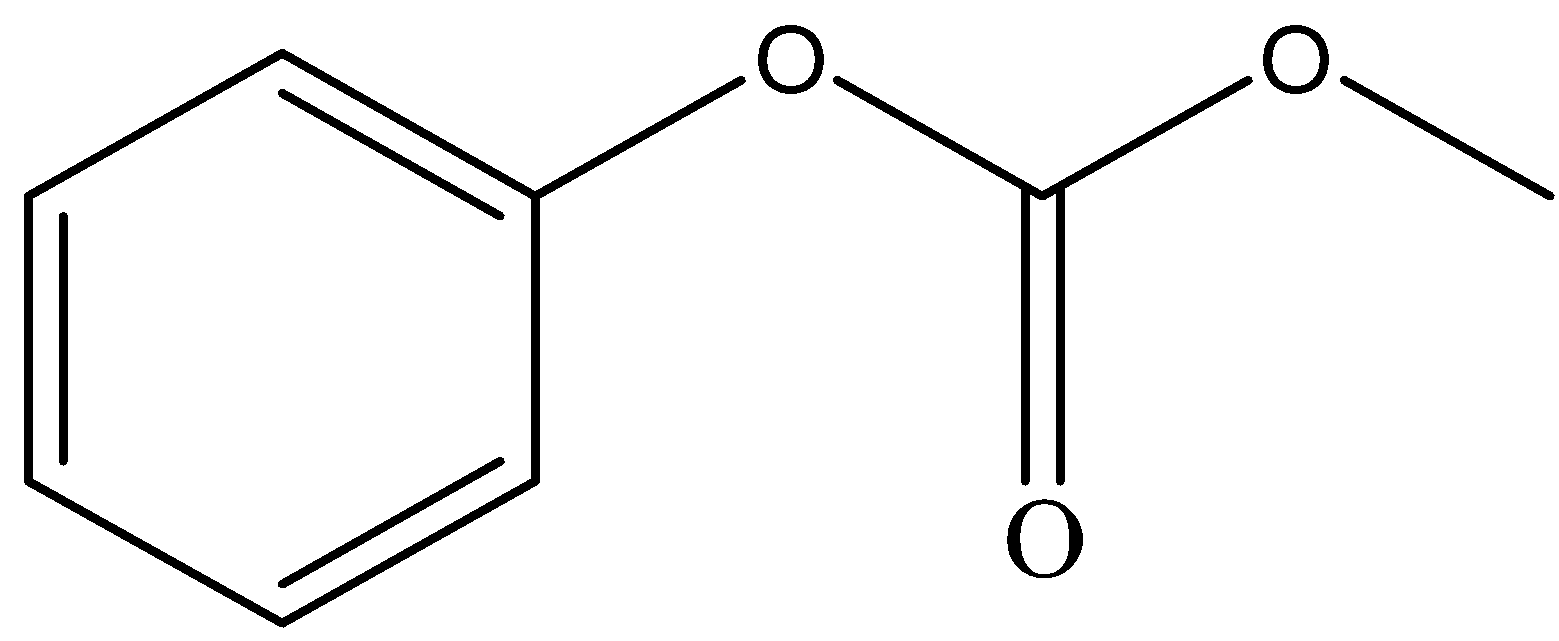 and
and 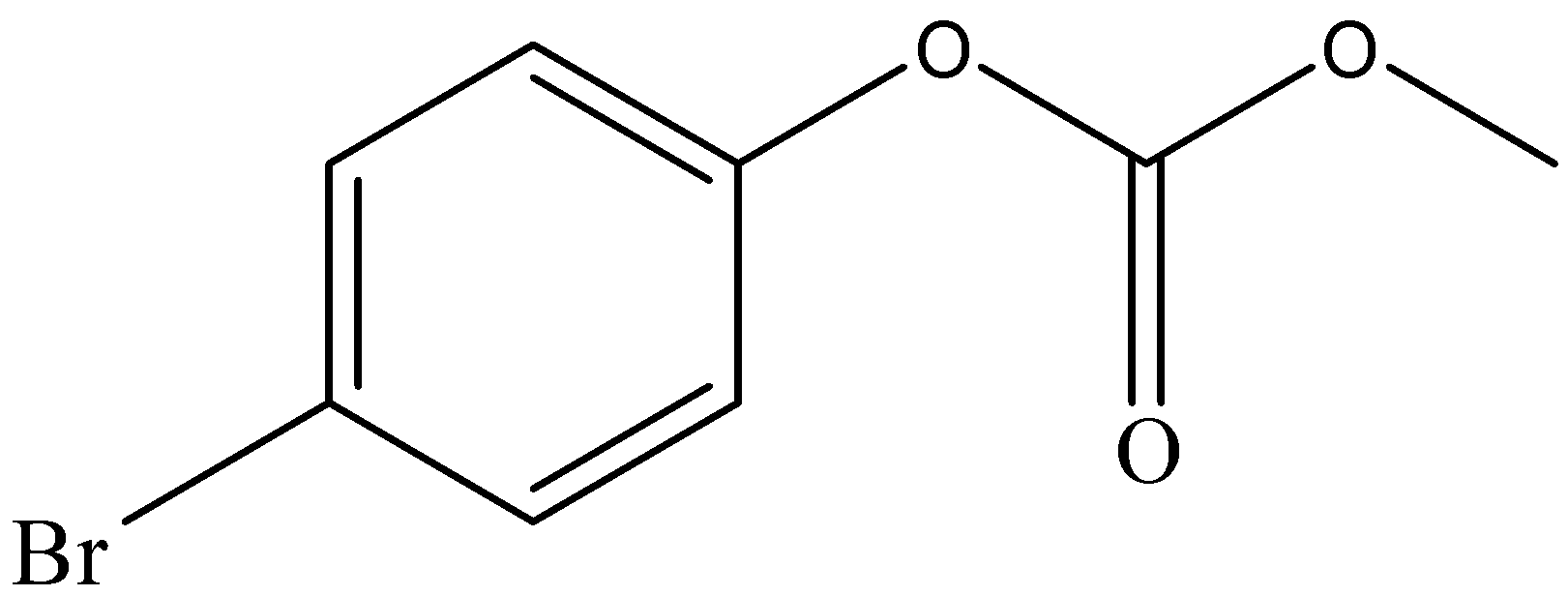
B.
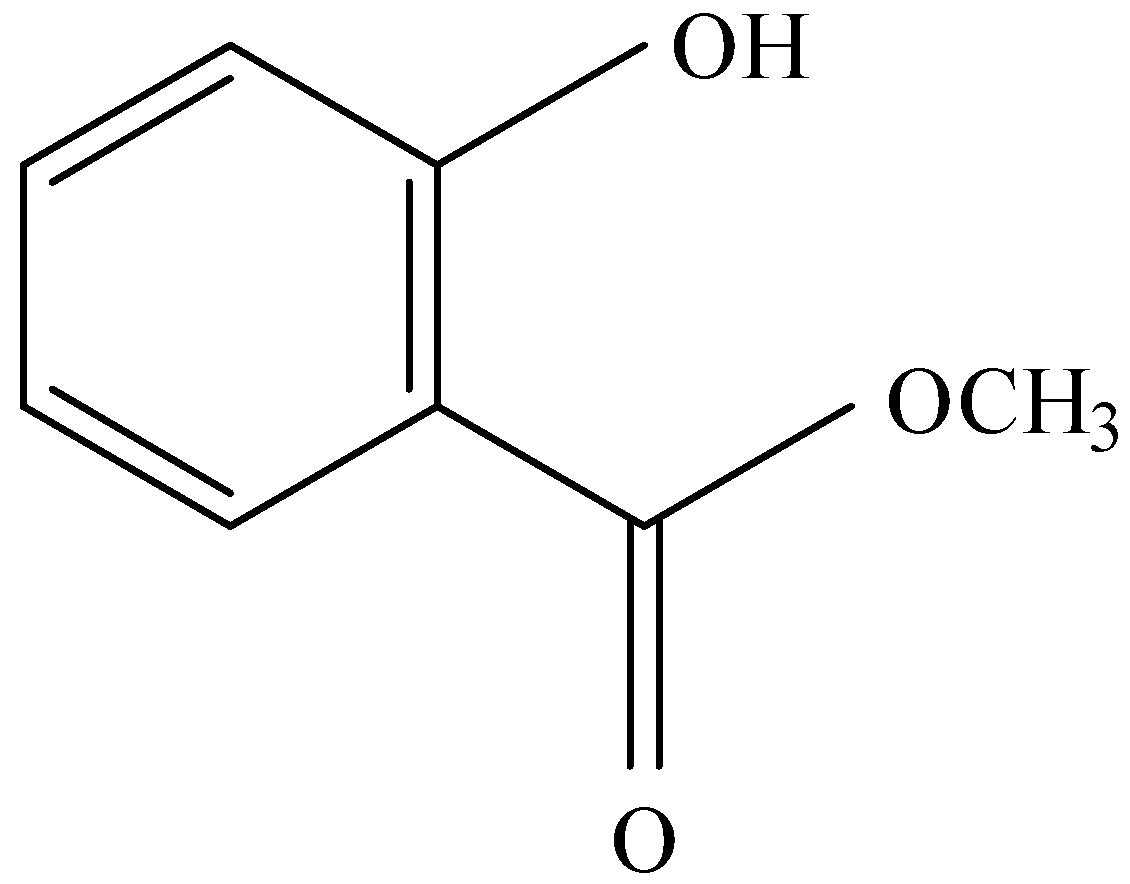 and
and 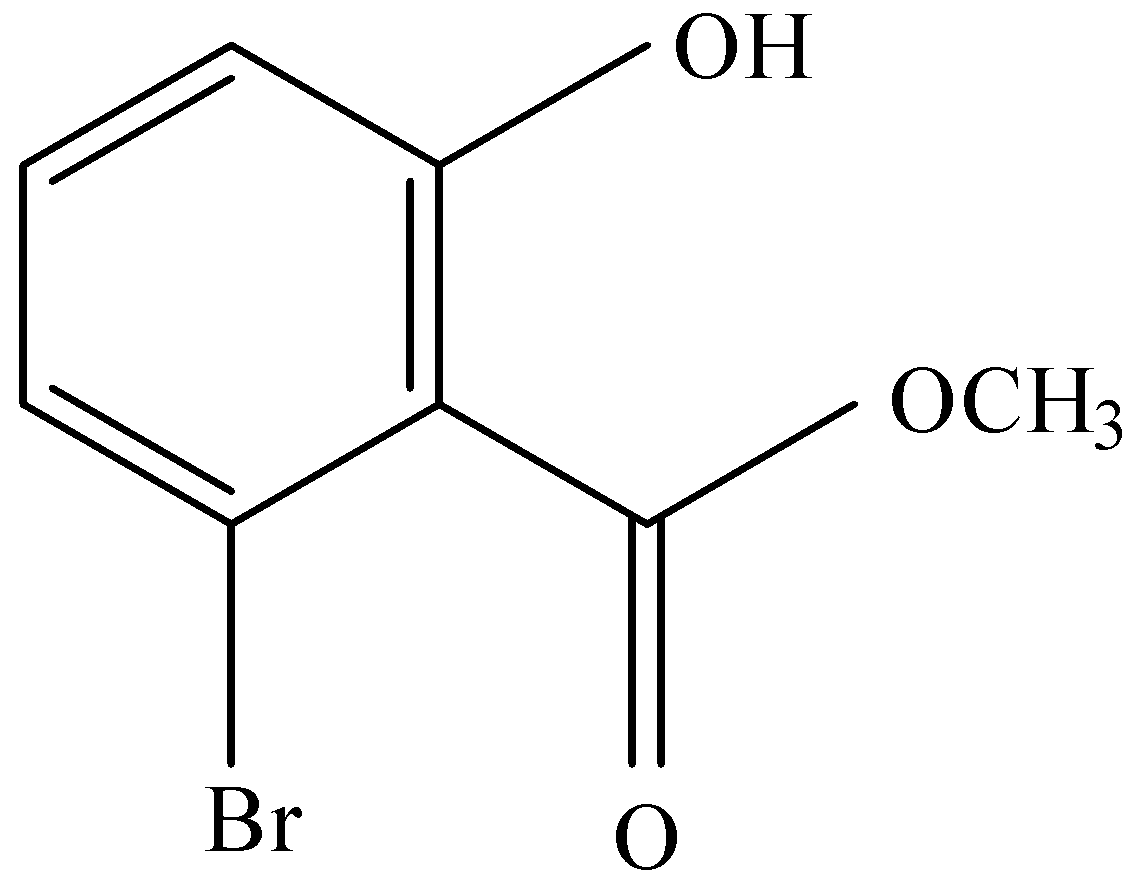
C.
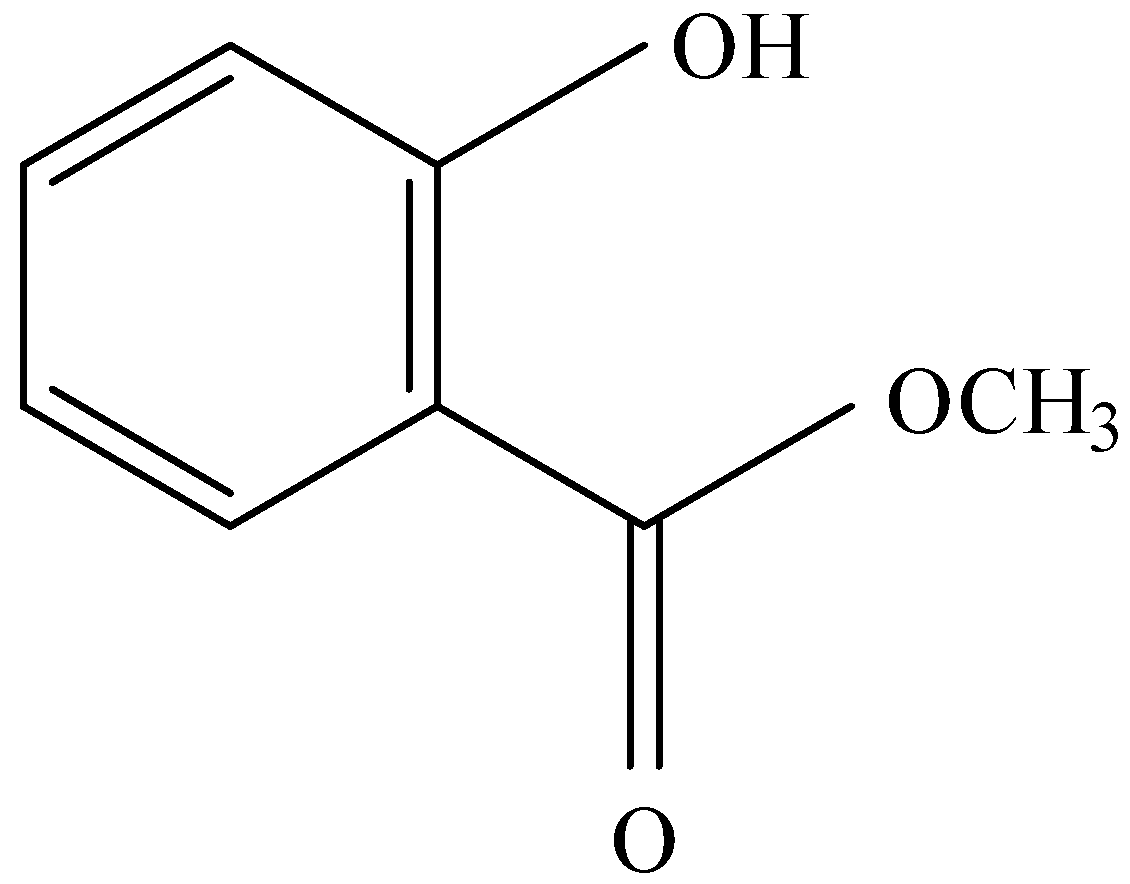 and
and 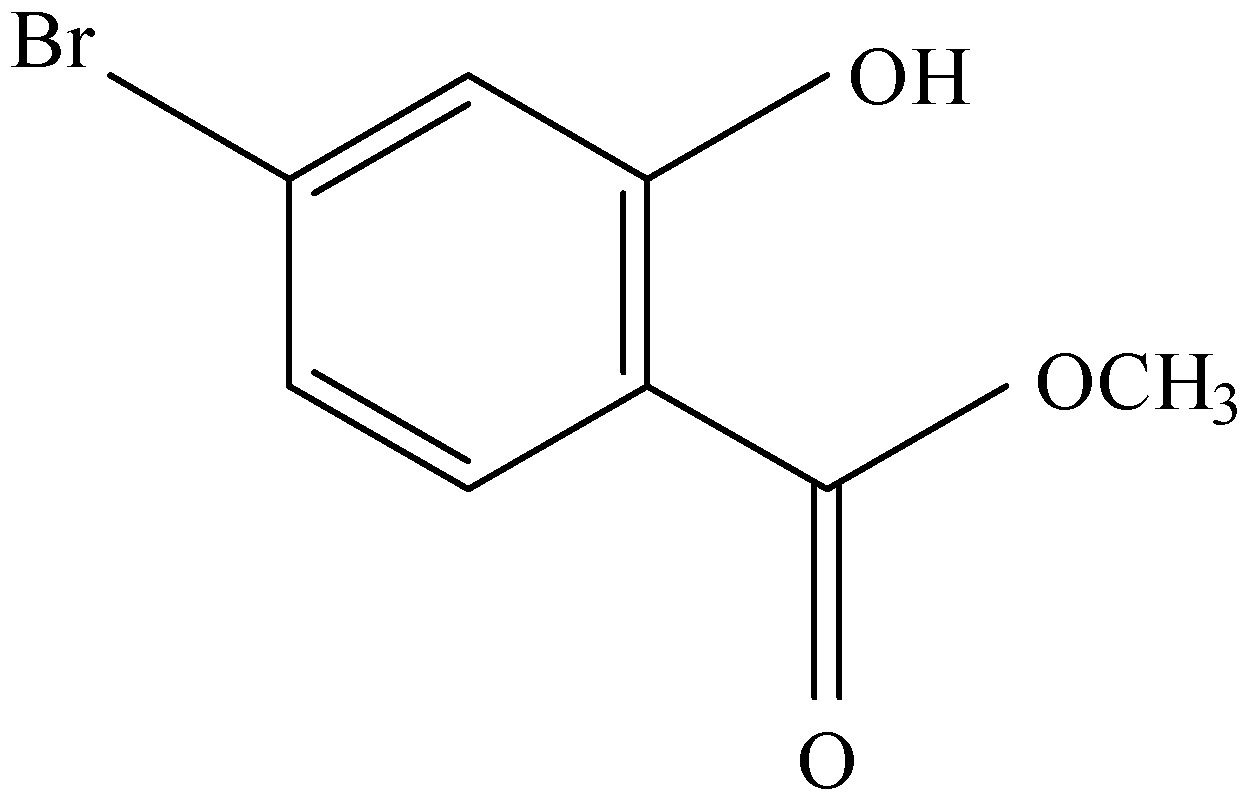
D.
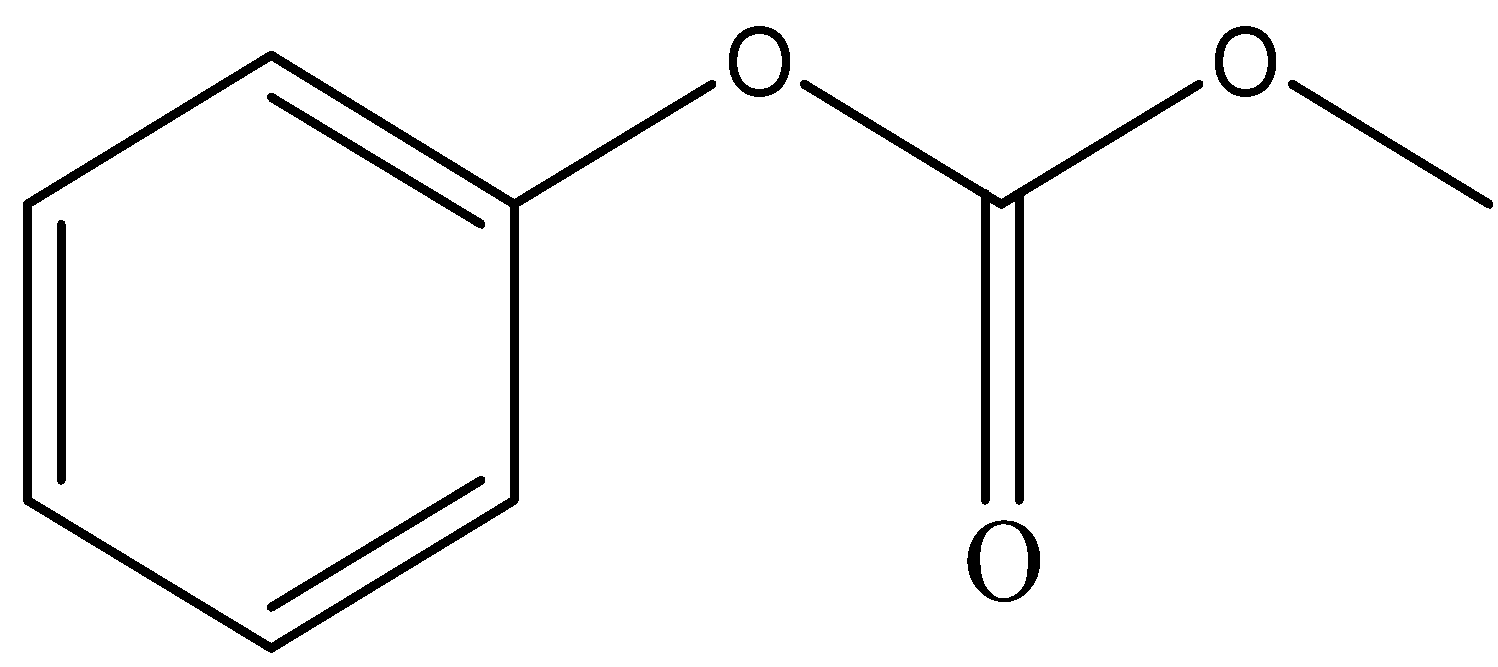 and
and 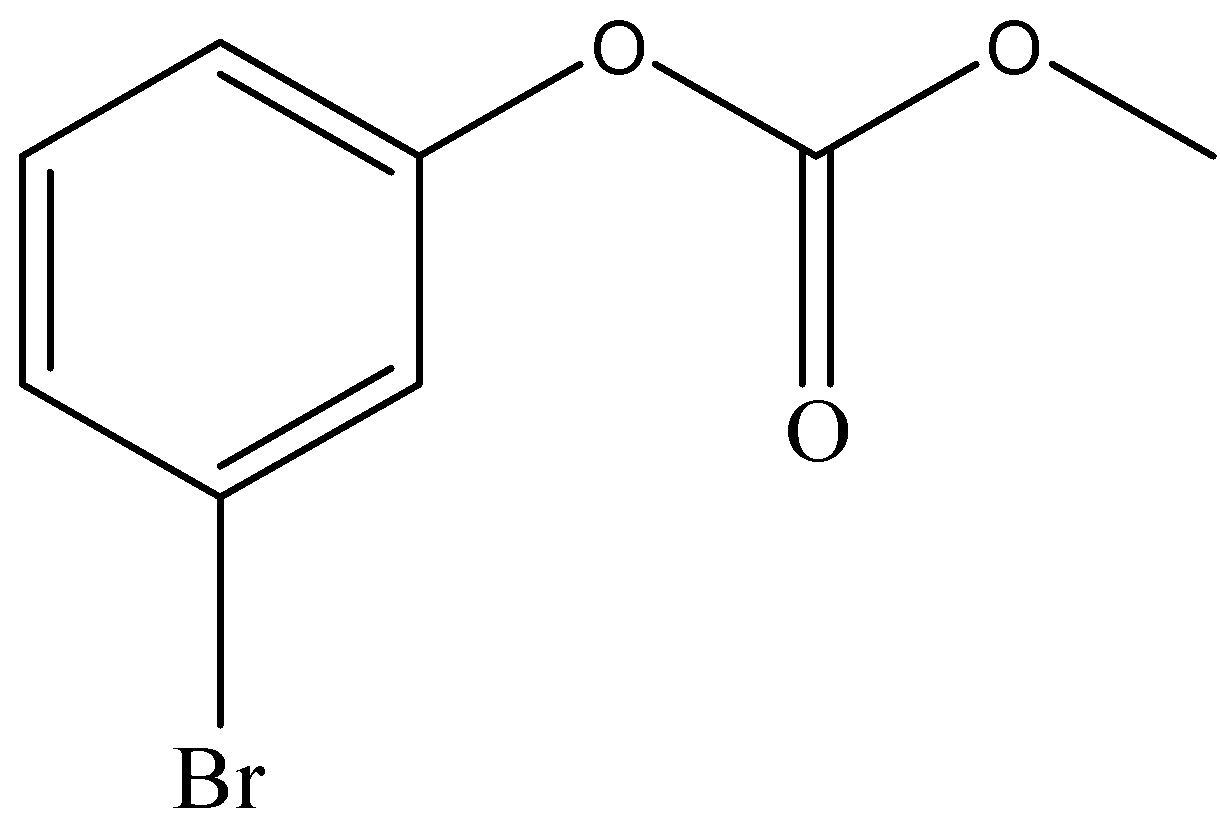
Solution
Here, in this reaction it is given that phenol reacts with methyl chloroformate which is a methyl ester of chloroformic acid in the presence of a strong base NaOH and it produces a product named A. The product A then reacts with Br2 to produce product B.
Complete step by step solution:
Sodium hydroxide, being a strong base, abstracts the proton from phenol to form phenoxide ion. Phenoxide ion, which is an electron rich species attacks the carbonyl carbon of methyl chloroformate which is an electron deficient carbon. This led to the removal of Cl− ion from methyl chloroformate. This leads to the formation of compound A.
After the formation of compound A, it reacts with Br2 to form compound B. The functional group of compound A, which is a para-directing group, pushes the electron density to para position. This leads the compound to attack Br2 through its para position to form compound B.
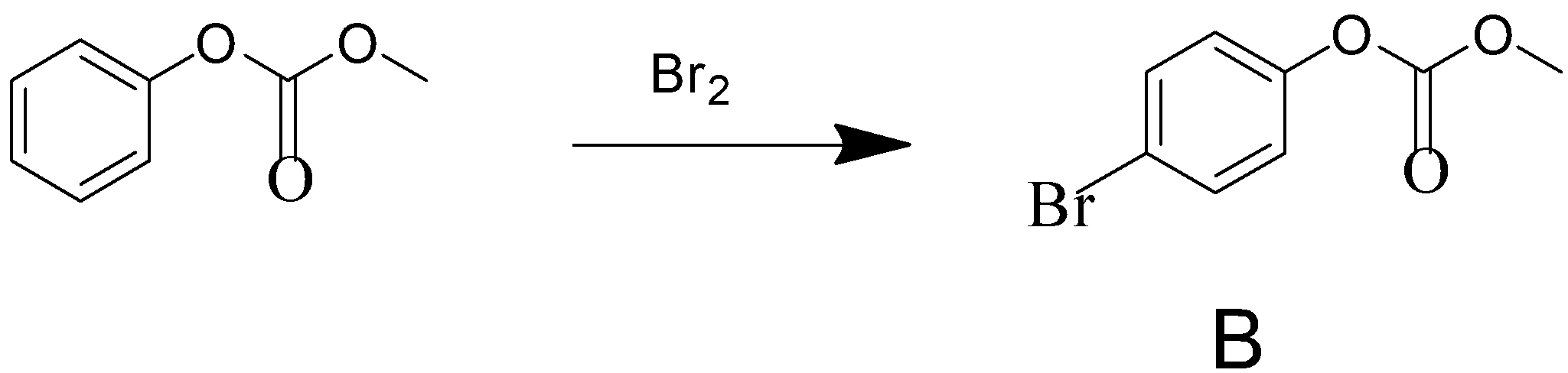
Hence, the option (a) is the correct answer.
Additional Information:
Methyl chloroformate is a common reagent used in organic synthesis whenever there is a need to introduce a methoxycarbonyl functional group to attack a nucleophile in a chemical reaction (called carboxymethylation). Methyl chloroformate is also generally used for the derivatization of functional groups such as carboxylic acids, , phenols and amines.
Methyl chloroformate is synthesised using methanol and phosgene.
COCl2+MeOH→ClCOOCH3+HCl
Note:
It is very important to note the nature of each of the compounds involved in the reaction. One should keep in mind that, in a chemical reaction, whenever there is possibility of an acid-base reaction to take place, we must make sure that it happens at first.
