Question
Question: Phenol on treating with concentrated \({H_2}S{O_4}\) at \(15 - {20^ \circ }C\) mainly produces: A:...
Phenol on treating with concentrated H2SO4 at 15−20∘C mainly produces:
A: phenol−2,4,6−trisulfonic acid
B: 2−hydroxy benzene sulphonic acid
C: phenol−4− sulphonic acid
D: a 50% mixture of ortho and para phenolsulphonic acid
Solution
In organic reactions, the temperature at which reaction is taking place and the concentration of reactants is very important. By changing the temperature or the concentration of reactants, the product is changed completely.
Complete step by step answer:
Chemical formula of phenol is C6H5OH. Phenol reacts with different concentrations of sulphuric acid at different temperatures to yield different products. When phenol is heated at 100∘C with concentrated sulphuric acid, 4−hydroxy benzene sulphonic acid is formed. Reaction taking place is:
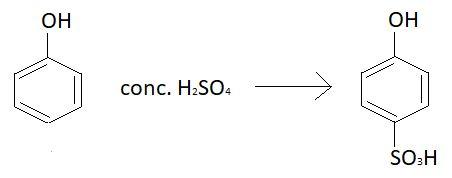
SO3H group is sulphonic acid group and OH group is hydroxyl group.
When phenol will react with concentrated sulphuric acid at 15−20∘C product will not be the same as above. At this temperature 2−hydroxy benzene sulphonic acid will be formed. Reaction involved at this temperature is:
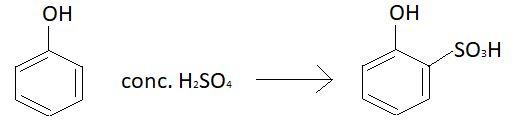
In this case sulphonic acid group will be attached with carbon atom at ortho position and hence the product formed will be 2−hydroxy benzene sulphonic acid.
So, the correct answer is Option C.
Additional Information:
Phenol reacts with dilute and concentrated nitric acid. Chemical formula of nitric acid is HNO3. In both the reactions products obtained are different. Phenol reacts with dilute nitric acid to give a mixture of ortho and para nitrophenol whereas phenol reacts with concentrated nitric acid to give picric acid or 2,4,6−trinitrophenol.
Note:
In benzene ring carbon atom which is present at adjacent position to carbon atom containing functional group is called ortho carbon, carbon which is opposite to carbon atom containing functional group is called para carbon and the carbon between ortho and para is called meta carbon.
