Question
Question: Phenol magnesium bromide reacts with methanol to give (1) A mixture of anisole and \(Mg(OH)Br\) ...
Phenol magnesium bromide reacts with methanol to give
(1) A mixture of anisole and Mg(OH)Br
(2) A mixture of benzene and Mg(OMe)Br
(3) A mixture of toluene and Mg(OH)Br
(4) A mixture of phenol and Mg(Me)Br
Solution
Recall the reaction of Grignard reagents with alcohols. General formula of Grignard reagent is RMgX. In the question, we are given phenol magnesium bromide which is a Grignard reagent and methanol, an alcohol.
Complete step by step solution:
We are given that phenol magnesium bromide, having chemical formula as C6H5MgBr, reacts with methanol (CH3OH).C6H5MgBr is a Grignard reagent, having general formula as RMgX where, R is any alkyl or aryl group and X is any halogen atom. Grignard reagents are good bases, that is, they abstract acidic hydrogen ions (H+). Acidic hydrogen is any hydrogen which is attached to a highly electronegative atom like oxygen. Alcohols (general formula: R′OH) contain acidic hydrogen.
General reaction of Grignard reagent with alcohol can be shown as:
R−MgX+R′−OH→R−H+Mg(R′)X
Here, methanol (CH3OH) has an acidic hydrogen, so phenol magnesium bromide (C6H5MgBr) i.e., a Grignard reagent will attack on the acidic hydrogen of methanol. Thus, the required chemical reaction by following the above general reaction can be represented as:
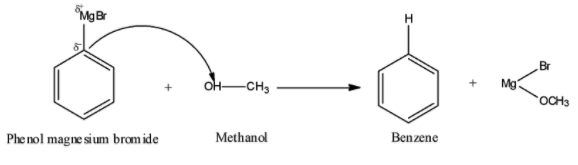
It is quite clear from the above reaction that when phenol magnesium bromide reacts with methanol, we get benzene (C6H6) and Mg(OMe)Br as products.
Thus, option (C) is the correct answer.
Note: Grignard reagents are generally formed by the reaction of magnesium metal with alkyl or aryl halides. It should be noted that Grignard reagents, besides being good bases, are also extremely good nucleophiles. As nucleophiles, they react with electrophiles such as carbonyl compounds (aldehydes, alcohols, esters, epoxides etc.). When Grignard reagent reacts with aldehydes and ketones, we get alcohols whereas when Grignard reagent reacts with alcohol, we get the corresponding alkane as a product.
