Question
Question: Phenol is: A.A base weaker than ammonia B.An acid stronger than carbonic acid C.An acid weake...
Phenol is:
A.A base weaker than ammonia
B.An acid stronger than carbonic acid
C.An acid weaker than carbonic acid
D.A neutral compound
Solution
To solve the above question we will compare basicity or acidity of phenol with ammonia and carbonic acid. We will use the basic chemical properties of phenol. Phenol is an aromatic organic compound with a molecular formula C6H5OH.
Complete answer:
Phenol is an organic hydroxy compound that consists of a benzene ring and a single hydroxy group as a substituent. The chemical structure of phenol is given below.
Now we will check all the options one by one. So let’s consider the first option (A) A base weaker than ammonia. We know that base is a substance that accepts hydrogen ions and releases hydroxide ions. Let’s understand it with the help of a reaction.
NH3+H2O⇆NH4++OH−
NH4+ The ammonium ion is formed along with the hydroxide ion. Oxygen is more electronegative than nitrogen. Ammonia is more basic than ammonia. So this potion is wrong.
Now for options (B) and (C), we will compare the acidic nature of phenol with carbonic acid. Carbonic acid is a chemical compound with a molecular formula H2CO3. So let’s try to understand it properly.
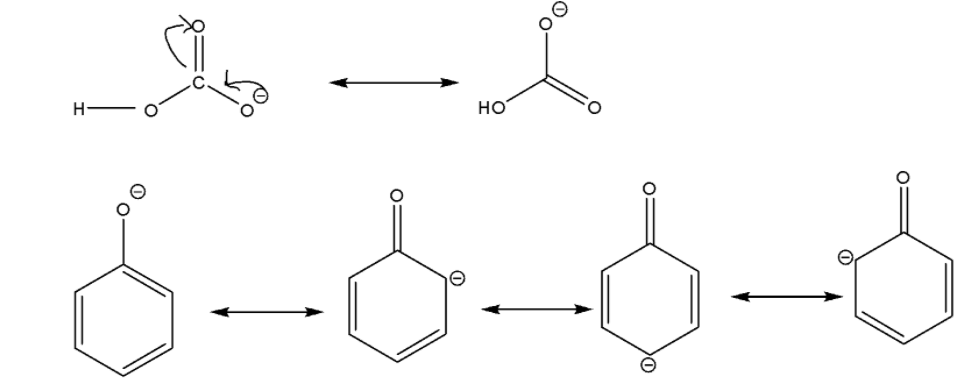
We can easily observe that carbonic acid forms bicarbonate ion and phenol forms phenoxide ion after losing H+ ion. Bicarbonate ions have two and phenoxide ions have four resonance structures. It will be easy for carbonic acid to lose H+ ion.
Now considering the last option (D) A neutral compound. We already observed that phenol is acidic.
We can conclude that phenol is an acid weaker than carbonic acid.
Therefore, the correct option is (C).
Note:
Resonance structures are the two examples of molecules in which the chemical interaction is the same but the electrons are distributed around the structure differently.
More the number of resonance structures formed, the more the stability of the chemical compound.
