Question
Question: Phenol does not decompose \[{\text{NaHC}}{{\text{O}}_{\text{3}}}\] to evolve \[{\text{C}}{{\text{O}}...
Phenol does not decompose NaHCO3 to evolve CO2but picric acid does.
A. True
B. False
Solution
Sodium bicarbonate (NaHCO3) is a weak base. In presence of acid NaHCO3 dissociate and liberate CO2gas. Draw the structures of both phenol and picric acid and determine their acid strength.
Complete step by step answer:
Sodium bicarbonate (NaHCO3) is a weak base when it reacts with acid it is converted into carbonic acid
(H2CO3). As carbonic acid is unstable it dissociates into CO2gas and water as follows:
NaHCO3 + H + →H2CO3→CO2+H2O
Now we will draw the structures of both phenol and picric acid and determine their acid strength as both are acidic in nature.
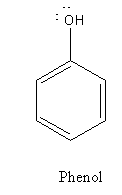
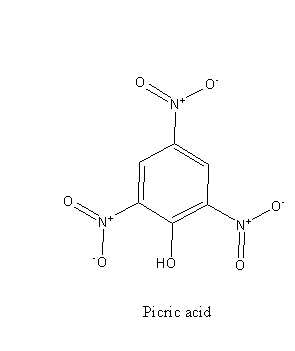
From the structure of phenol and picric acid we can say that picric acid is substituted phenol, there are three electron-withdrawing - NO2 groups bonded to the ring.
The acidic strength of substituted phenol is dependent on the nature of the substituent.
The electron-withdrawing group decreases the electron density of the phenol ring and stabilizes the negative charge and thus increases the acid strength.
In the case of the picric acid, the substituent is a nitro group ( - NO2 ). It is an electron-withdrawing group so picric acid is a stronger acid than phenol.
As phenol is weak acid it cannot react with NaHCO3 to evolveCO2.
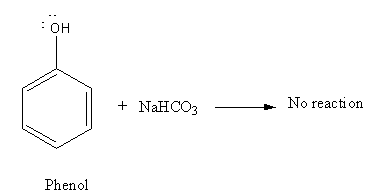
However, picric acid is stronger acid which reacts with base as shown below:
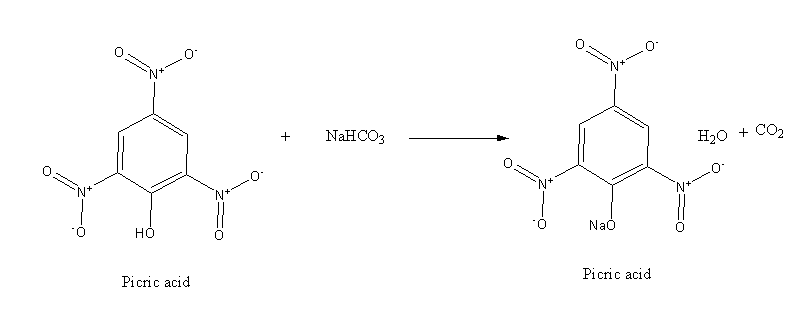
Hence, the given statement is true i.e, option ‘A’ is the correct answer.
Note: Ring activating groups that are electron-donating groups, decrease the strength of acid while a ring deactivating group increases the strength of the acid. So while determining acid strength it is necessary to determine whether the substituent is an electron-donating group or electron-withdrawing group.
