Question
Question: Phenol can be distinguished from alcohol by which reagent: (a) \(Na\) (b) Alcoholic \(FeC{l_3}\...
Phenol can be distinguished from alcohol by which reagent:
(a) Na
(b) Alcoholic FeCl3
(c) Br2/HOH
(d) NaOH
Solution
We have to remember that the phenols are just like alcohols but they have higher boiling points and are highly soluble in water than alcohols. Phenols form strong Hydrogen – Hydrogen bonds. Alcohols are acidic in nature, they react with metals to form alkoxides.
Complete answer:
When phenol reacts with alcoholic FeCl3 , it will produce a violet color.
We can draw the chemical reaction for this reaction as,
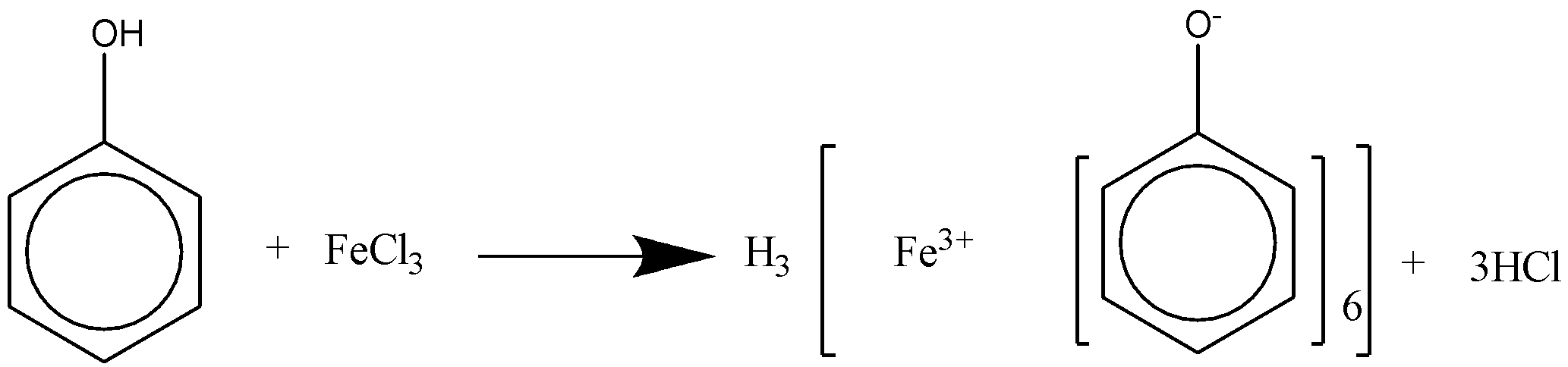
As phenols are acidic in nature when it reacts with alcoholic FeCl3, it forms a violet colored complex. The alcohol does not react with the compound as it is neutral when reacted to alcoholic FeCl3. If we increase the temperature of the reaction vigorously then we can expect some result of reaction between alcohol and FeCl3 which is practically difficult to achieve. Though the distinguishing between the two compounds phenol and alcohol can be contradictory in many cases, as both the compounds react with many reagents.
But here specifically in this case the alcohol does react with other compounds as all the other compounds are basic in nature. While alcohol is generally neutral in nature. Hence the reaction is not possible. Though phenol also is a type of alcohol but it is more acidic than alcohol in comparison. This type of reaction is generally used to detect whether there is phenol compound present in the composition or not. If by adding alcoholic FeCl3 it gives a violet colored compound, then it implies that the solution contains phenol.
Therefore, the option B is correct.
Note:
We can detect Phenols by various other tests like,
-Litmus Test – Litmus paper is used to test whether the solution is acidic, basic or neutral. Phenols turn blue litmus paper red, because they are acidic in nature.
-Libermann’s Test – In this test the given solution reacts in the presence of Sulfuric acid and sodium nitrate. Phenols react with this reagent to form a yellow compound also called quinone monoxime complex.
-Bromine Water Test – Phenol reacts with bromine to form white precipitate of tribromophenol.
-Phthalein Dye Test – Phenol when reacted with phthalic anhydride on heat in the presence of sulfuric acid, it forms a colorless compound phenolphthalein. Further reacting it with a dilute sodium hydroxide solution, it gives a fluorescent pink color compound called fluorescein.
