Question
Question: Phenol can be converted into anisole by: (A) \(C{{H}_{2}}{{N}_{2}}\) / ether (B) \({{(C{{H}_{3}}...
Phenol can be converted into anisole by:
(A) CH2N2 / ether
(B) (CH3)2SO4 / NaOH
(C) CH3I
(D) All of these
Solution
An attempt to this question can be made by determining the action of the reagents mentioned in the options. Draw the expanded structure of the starting compound and the final product i.e. phenol and anisole. Now determine the reagent that can possibly give the desired product. Based on this you can answer the question.
Complete step by step answer:
We will draw the expanded structure of the starting compound and the final product i.e. phenol and anisole as suggested in the hint.
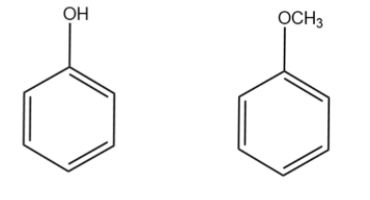
PhenolAnisole
We will determine the reaction of phenol with the reagents mentioned in the options.
(A) CH2N2 / ether
Diazomethane in the presence of ether converts alcohols into ethers. This is because the −CH2 group attaches to the alcohol group releasing molecular nitrogen. Thus, phenol when reacted with CH2N2 / ether produces the ether anisole.
(B) (CH3)2SO4 / NaOH
Dimethyl sulfate is a reagent whose behavior is similar to sulfuric acid. The only difference is instead of hydrogen ions the compound releases CH3+ ions in the solution. These ions, when reacted with phenol, form an ether. The ether is called anisole.
(C) CH3I
Methyl iodide, like dimethyl sulfate, releases CH3+ ions in the solution. Thus CH3I also converts phenol into anisole.
From the above statements we can conclude that all the three reagents convert phenol into anisole.
Therefore, the correct answer is option (D) i.e. all of these.
Note: It is important to know that along with dimethyl sulfate we use sodium hydroxide to enhance the rate of the reaction.
