Question
Question: \( Phenol \) , \( {C_6}{H_5}OH \) , is allowed to react with \( diazomethane \) , \( C{H_2}{N_2} \) ...
Phenol , C6H5OH , is allowed to react with diazomethane , CH2N2 . The product formed is
(A) C6H5CH2OH
(B) C6H5OCH3
(C) 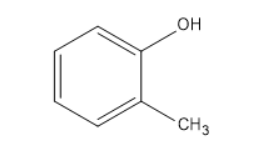
(D) 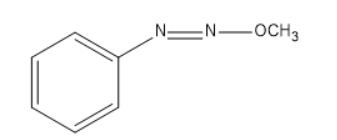
Solution
Diazomethane is a very reactive compound. It acts as a methylating agent. Diazomethane converts alcohol into ethers in the presence of ether. To solve this question remember the fact that Diazomethane is an alkylating agent therefore alkylates the phenol.
Complete answer:
Phenol is an aromatic organic compound which is a volatile compound. Phenol has a widespread use involving as a disinfectant, mouthwash, medicinal use like that in surgical antiseptics. Phenol are similar to alcohols but they form stronger hydrogen bonds that is why they are more soluble in water than are alcohols. Phenol was first mined from coal tar, but today it is manufactured on a large scale from petroleum. It is an important industrial product as a pioneer to various materials and useful compounds. Phenol and its chemical products are very important for the production of Bakelite, polycarbonates, detergents, nylon, epoxies, herbicides such as phenoxy herbicides.
Diazomethane is the simplest diazo compound. As mentioned in the hint Diazomethane converts alcohol into ethers. This is due to the fact that −CH2 group attaches to the alcohol group releasing molecular nitrogen.
Diazomethane when reacting with a Phenol acts as a base and abstracts a proton from phenol. This results in the formation of phenoxide ions. After this phenoxide ion acts as a nucleophile and under this nucleophilic attack Anisole ether is formed.
The reaction is shown below:
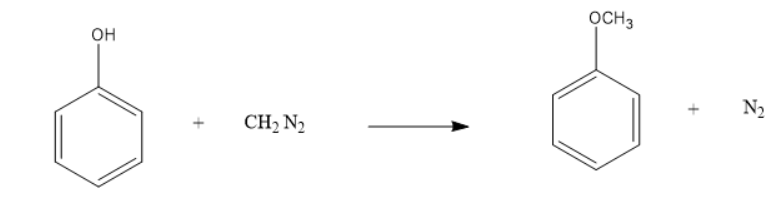
Anisole is formed in the reaction and nitrogen gas is liberated when Diazomethane reacts with . Phenol . This reaction takes place in the presence of Fluoroboric acid .
Anisole or methoxybenzene is an organic compound which is a colourless liquid. Its smell is reminiscent of anise seed. Due to this smell it is used in making perfumes, flavouring agents as well as solvent.
Note:
We not only use Fluoroboric acid to enhance the rate of reaction but also chemicals like Sodium hydroxide which increases the rate of reaction. During such reactions, liberation of nitrogen always takes place.
