Question
Question: \[Ph-C\equiv CH\xrightarrow[MeOH]{Me{{O}^{-}}}(A)\]Major product of the reaction is: (A) Major product of the reaction is:
(A) 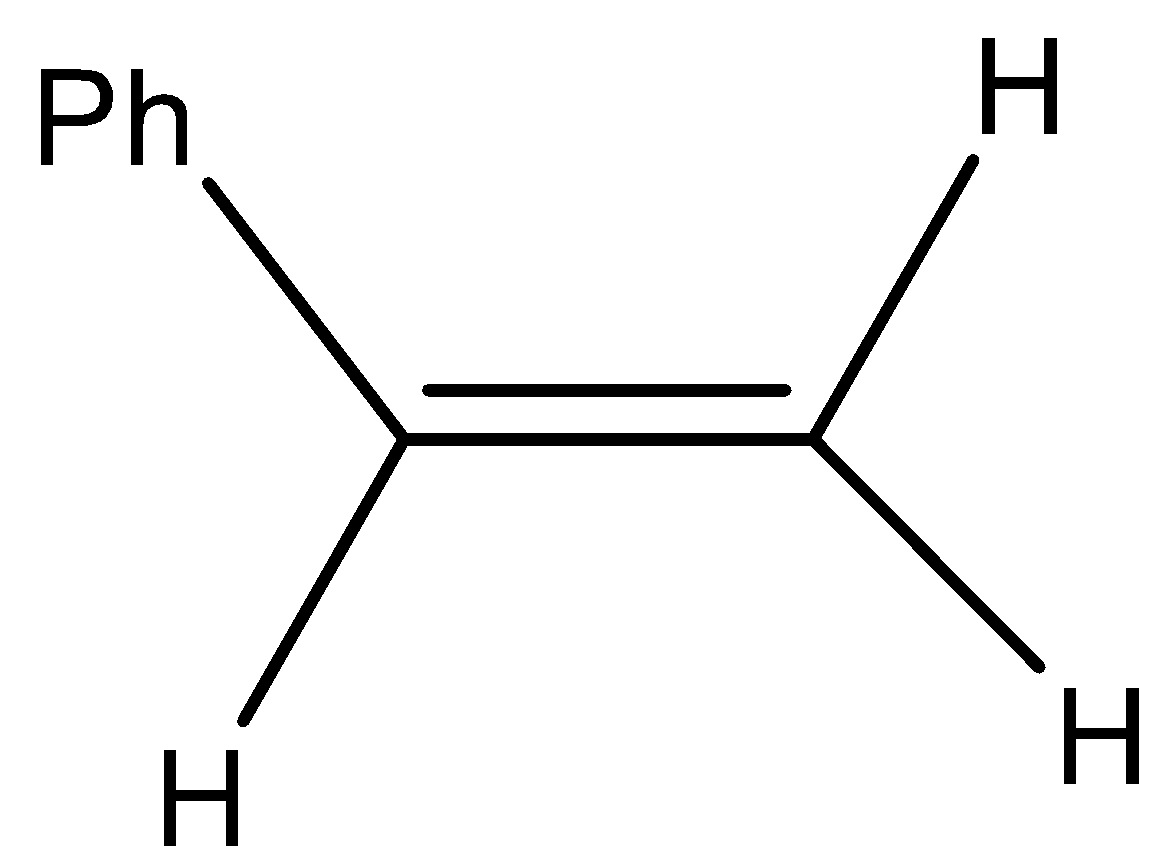
(B) 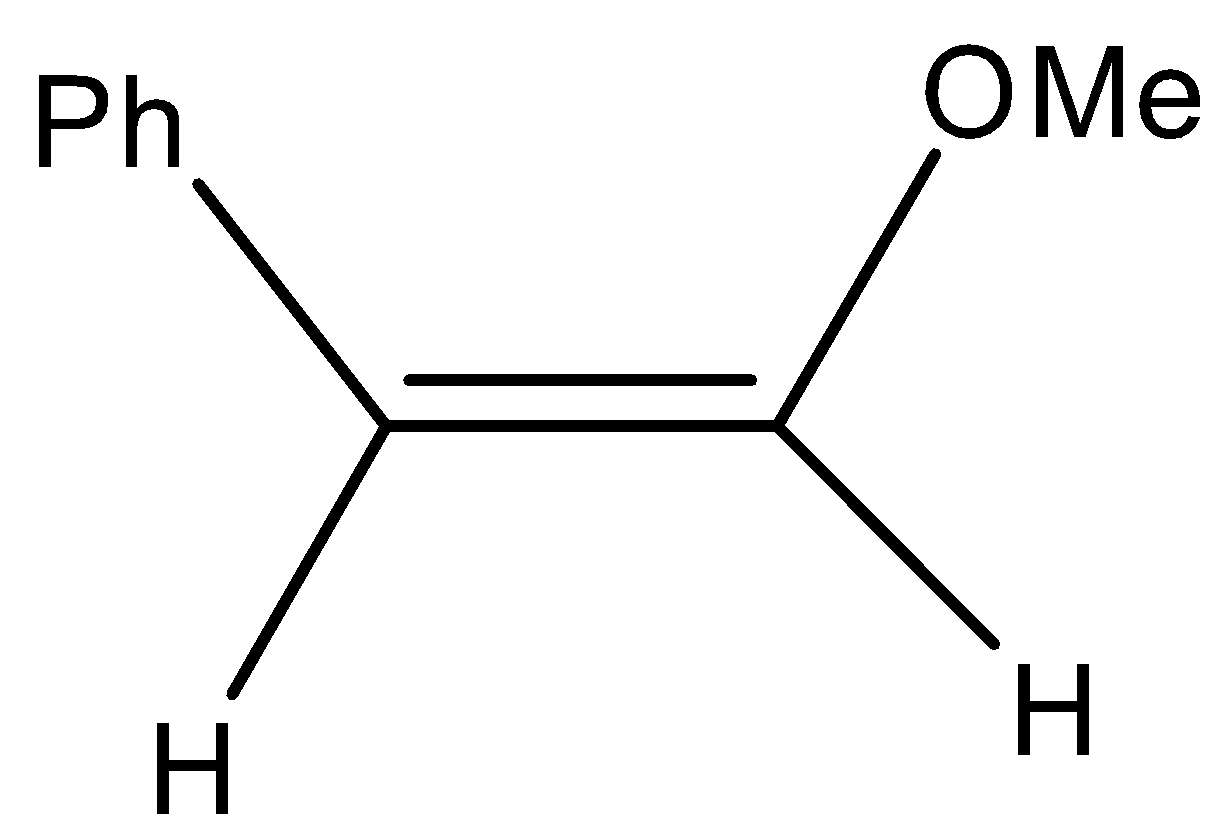
(C) Ph−C≡C−OMe
(D) 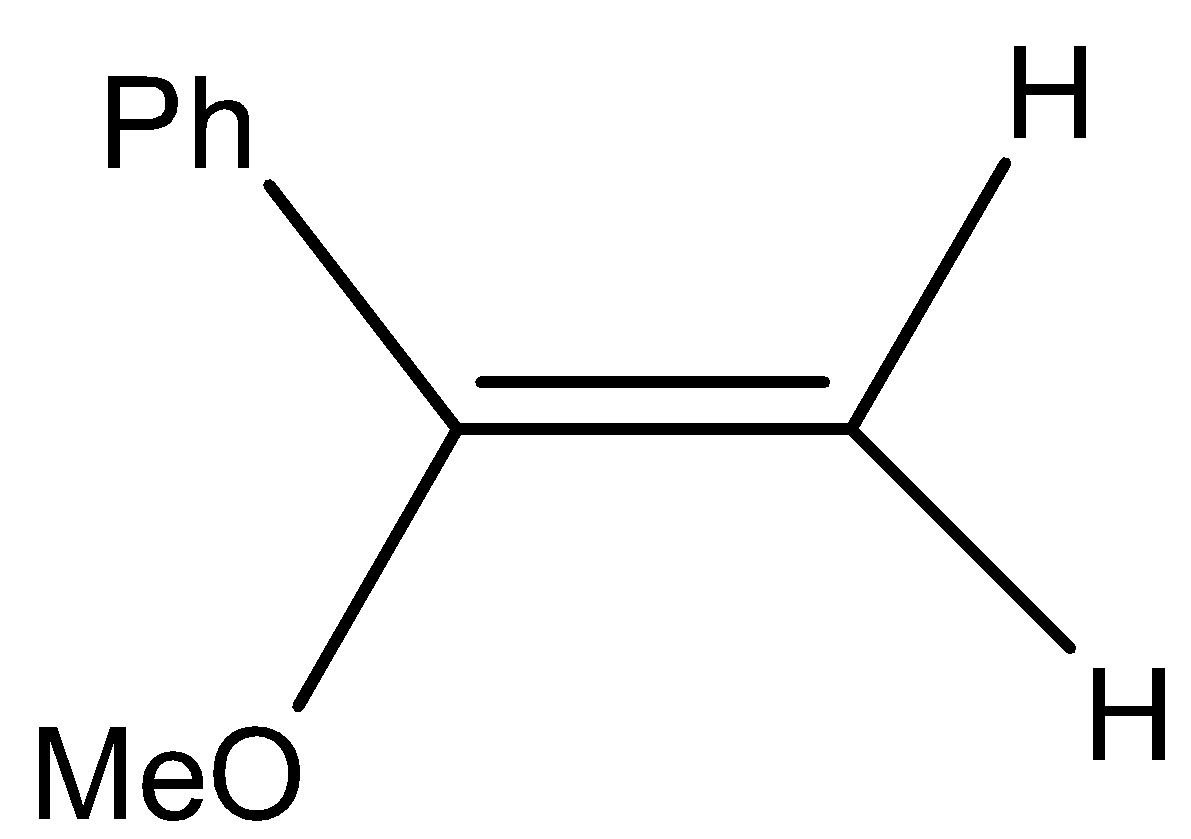
Solution
The addition of compounds to acidic hydrogens containing acetylene in presence of base is called vinylation. Because vinyl derivatives are the products formed in the base catalyzed reactions. It is an example for additional reactions on acetylene.
Complete step by step answer:
-The given reactant in the reaction is phenyl acetylene.
-In the given reaction methoxide ion is going to react with the phenyl acetylene.
-When methoxide ion is going to react with phenyl acetylene there is a chance of formation of two compounds as the products.
-The complete reaction of phenylacetylene with methoxide ion is as follows.
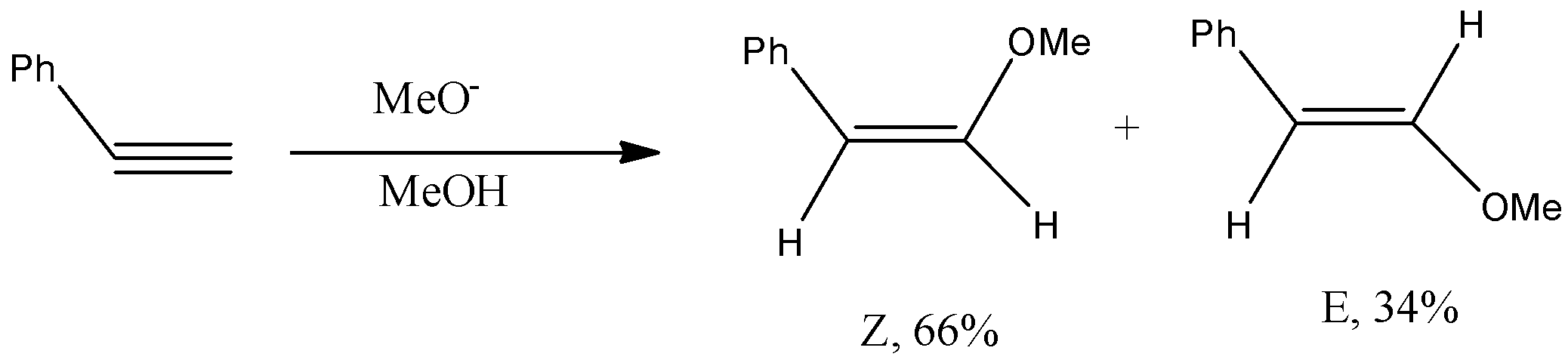
-In the above reaction phenyl acetylene undergoes Anti-Markovnikov addition with methoxide ion.
-The two products formed in the reaction are Z and E forms.
-The Z-isomer formed in 66% yield and 34% yield of E-isomer.
-Here Z means cis isomer and E means trans isomer.
-The major product formed in the above reaction is z form or cis isomer.
-Therefore the major product formed is
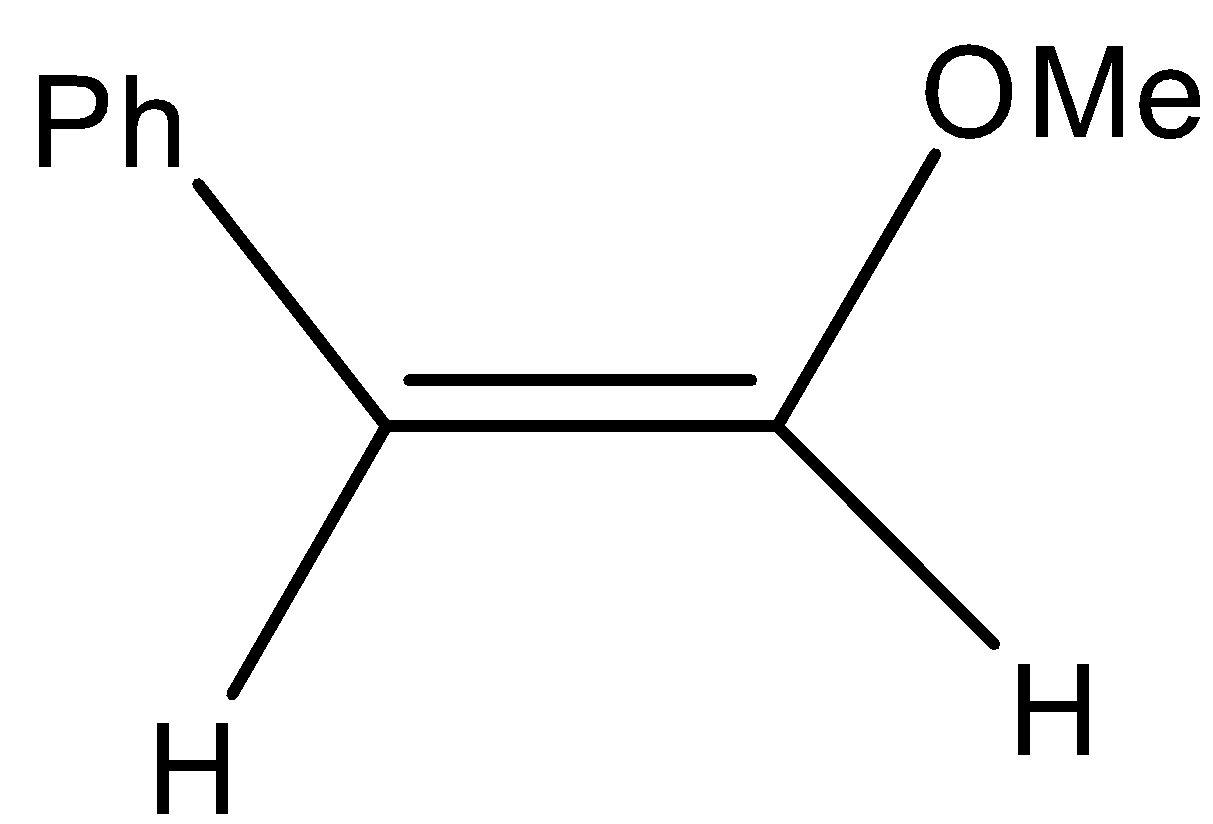
-So, the correct option is B.
Additional information:
-The name of the product which is formed in the given reaction is β-methoxystyrene.
-The given reaction is second order, first order with respect to phenylacetylene and first order in respect to methoxide ion and zero order with respect to methanol.
Note: In the given reaction the reactant undergoes Anti-Markovnikov rule means the methoxide is going to add where a high number of hydrogens is there or where the carbon is less substituted. This type of reaction is called regioselective reactions.
