Question
Question: Peroxy linkage is present in: (A) Caro’s acid (B) Pyrosulfuric acid (C) Sulfurous acid (D) D...
Peroxy linkage is present in:
(A) Caro’s acid
(B) Pyrosulfuric acid
(C) Sulfurous acid
(D) Dithionic acid
Solution
If the compound has two oxygen atoms bonded by a single bond, then the compound has a peroxy linkage. The Peroxy linkage is represented as −O−O−.
Complete step by step solution:
If the compound has two oxygen atoms bonded by a single bond, then the compound has peroxy linkage. The Peroxy linkage is represented as −O−O−.
So, let us see the structures of all the options one by one:
(A)- Caro’s acid: Caro’s acid is also known as Peroxomonosulfuric acid. This is an oxoacid of the sulfur element. The formula of Caro's acid is H2SO5. The structure of Caro’s acid is given below:
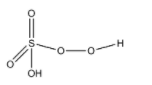
This compound has one peroxy linkage.
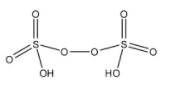
(B)- Pyrosulfuric acid: Pyrosulfuric acid is also known as oleum. It is also an oxoacid of the sulfur element. The formula of Pyrosulfuric acid is H2S2O7. The structure of Pyrosulfuric acid is given below:
This compound doesn’t have a peroxy linkage.
(C)- Sulfurous acid: Sulfurous acid is an element that has a lone pair on the sulfur element. It is also an oxoacid of the sulfur element. The formula of Sulfurous acid is H2SO3. The structure of Sulfurous acid is given below:
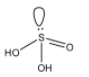
This compound doesn’t have a peroxy linkage.
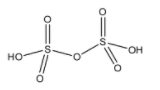
(D)- Dithionic acid: Dithionic acid is an oxoacid of the sulfur element. The formula of Dithionic acid is H2S2O6. The structure of Dithionic acid is given below:
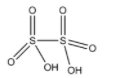
This compound doesn’t have a peroxy linkage.
Therefore, the correct answer is an option (a)- Caro’s acid.
Note: Marshall’s acid or Peroxodisulfuric acid is also an oxoacid of the sulfur element. The formula of Marshall's acid is H2S2O8. Other than Caro’s acid, Marshall’s acid is the compound of sulfur which has peroxy linkage. The structure is given below: