Question
Question: Perform the following conversion 1\. p-fluorotoluene to p-fluorobenzaldehyde 2\. Allyl alcohol t...
Perform the following conversion
1. p-fluorotoluene to p-fluorobenzaldehyde
2. Allyl alcohol to propenal.
Solution
Hint : p-fluorotoluene is converted to p-fluorobenzaldehyde by 3 different reactions in different conditions with the help of different reagents while Ally alcohol is converted to propenal on reaction with pyridinium chlorochromate.
Complete Step By Step Answer:
p-fluorotoluene is an organic compound with −CH3 and F group on the para position of the benzene ring. It can be converted into p-fluorobenzaldehyde by substituting the methyl group to −CHO group via three different conversions.
First, we will convert p-fluorotoluene to p-fluorobenzyl chloride via free radical halogenation.
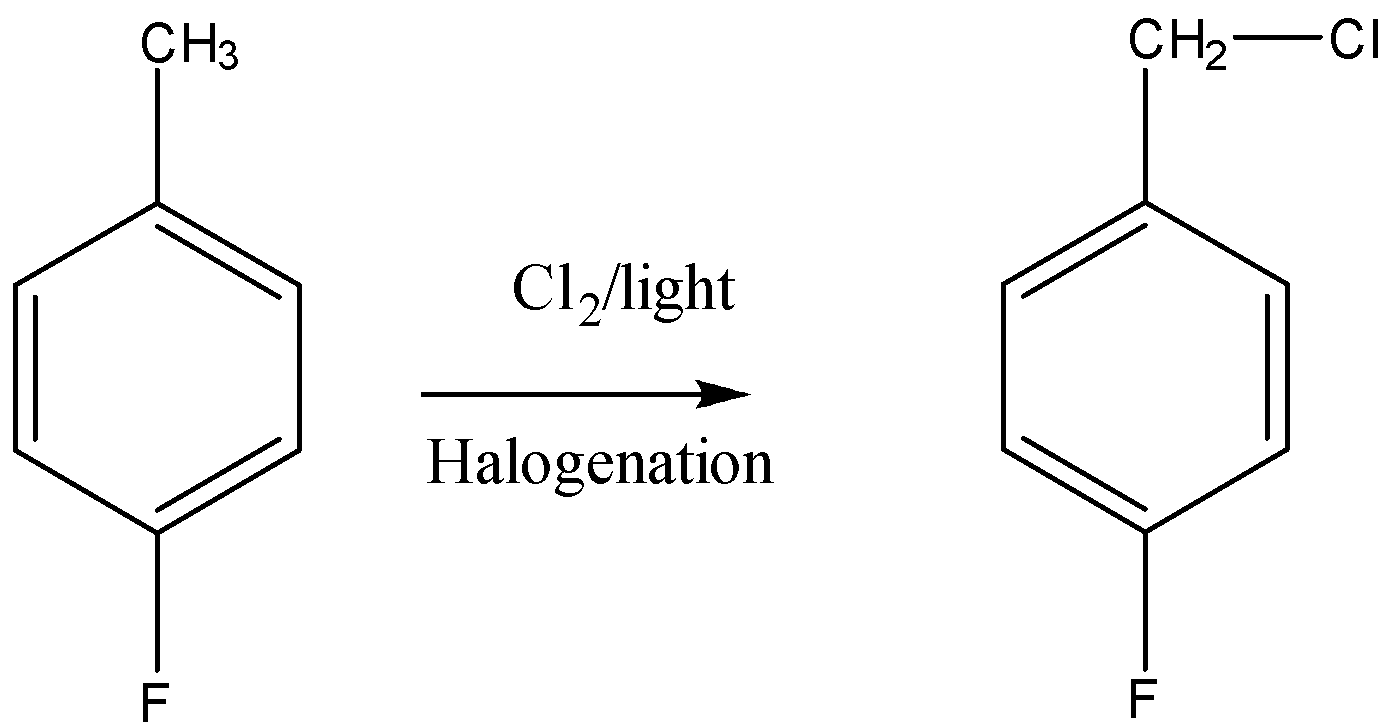
Now, the p-fluorobenzyl chloride will be converted to hydroxyl group by eliminating the −Cl with the help of thionyl chloride.
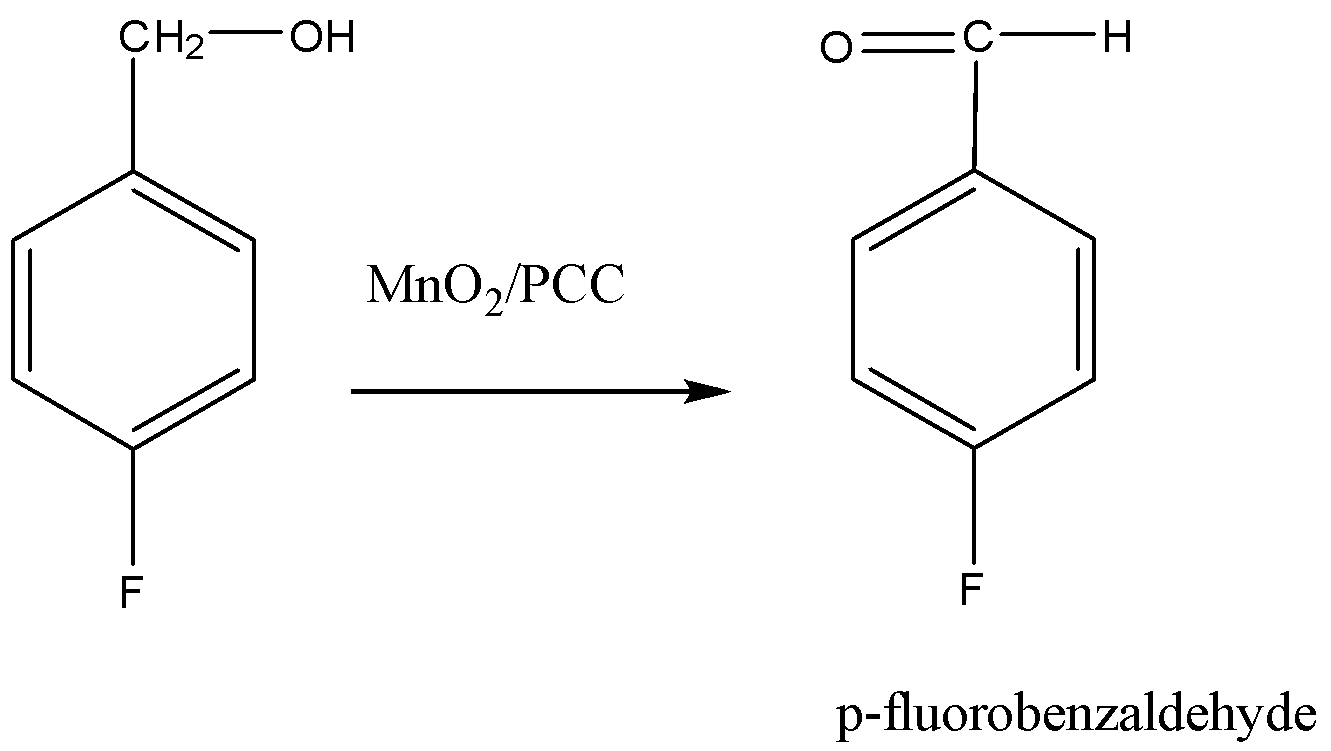
Alcohols are oxidized into aldehyde in the presence of a strong oxidizing agent. Now, the p-fluorobenzyl alcohol will be converted to p-fluorobenzaldehyde by oxidizing the −OH with the help of Manganese oxide in the presence of PCC.
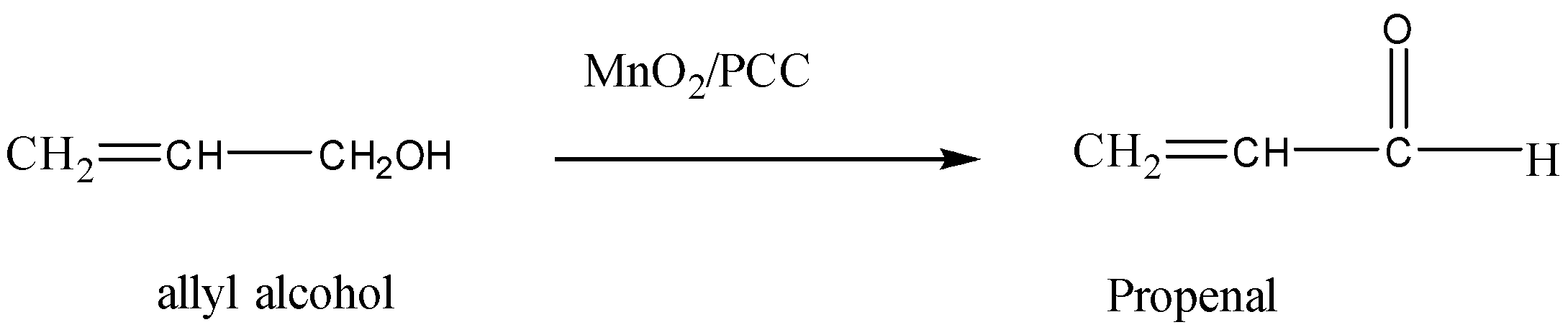
Allyl alcohol is converted into propenal when it is oxidised in the presence of a strong oxidising agent like MnO2 in the presence of PCC . Propenal is unsaturated aldehyde also known as acrolein.
Additional Information:
Acrolein is a simple unsaturated aldehyde. It is a colourless liquid having a piercing and an acrid smell. Its major use is as a contact herbicide to control the submerged and floating weeds and algae in irrigation canals. It is also used at 10 ppm in irrigation and recirculating waters. It can also be used as a biocide in drilling waters in the oil industry.
Note :
p-fluorotoluene is converted to p-fluorobenzaldehyde via free radical halogenation then it is reacted in presence of thionyl chloride to get p-fluorobenzyl alcohol and then alcohol is oxidised to aldehyde. The same type of oxidation is done in case of allyl alcohol to get propenal.
