Question
Question: Perdisulphuric acid, H₂S₂O₈ can be prepared by electrolytic oxidation of H₂SO₄, oxygen and hydrogen ...
Perdisulphuric acid, H₂S₂O₈ can be prepared by electrolytic oxidation of H₂SO₄, oxygen and hydrogen gases are by products. In such an electrolysis, 9.08 L of H₂ and 2.27 L of O₂ were generated at STP. What is the mass of H₂S₂O₈ formed?
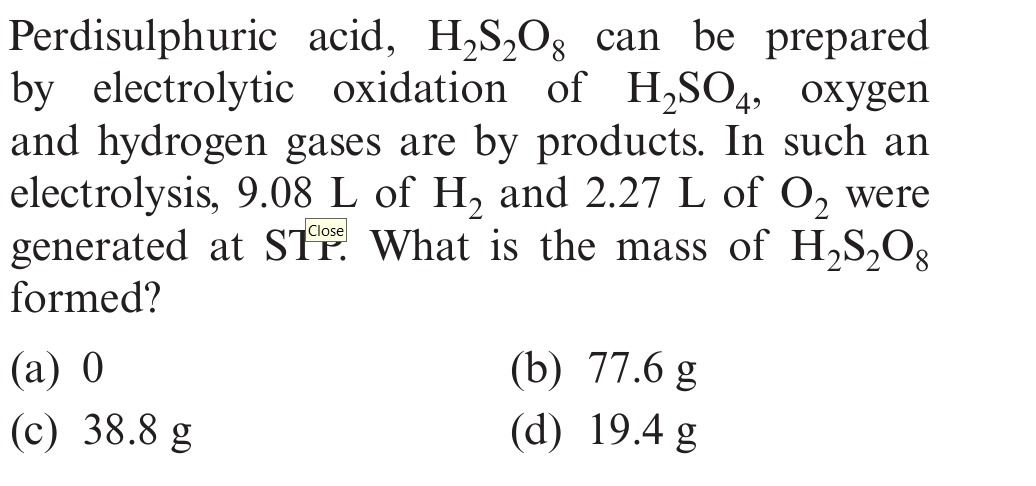
0
77.6 g
38.8 g
19.4 g
38.8 g
Solution
The electrolysis process involves the following electrode reactions: Anode:
- Formation of perdisulphuric acid: 2H2SO4→H2S2O8+2H++2e−
- Formation of oxygen gas: 2H2O→O2+4H++4e− Cathode:
- Formation of hydrogen gas: 2H++2e−→H2
Let nH2S2O8, nO2, and nH2 be the moles of H₂S₂O₈, O₂, and H₂ produced, respectively. The number of moles of electrons transferred for each process are:
- For H₂S₂O₈: 2×nH2S2O8
- For O₂: 4×nO2
- For H₂: 2×nH2
The total electrons produced at the anode must equal the total electrons consumed at the cathode. Electrons from anode = Electrons for H₂S₂O₈ formation + Electrons for O₂ formation Electrons for cathode = Electrons for H₂ formation
Thus, 2×nH2S2O8+4×nO2=2×nH2. Dividing by 2, we get: nH2S2O8+2×nO2=nH2. Rearranging to find moles of H₂S₂O₈: nH2S2O8=nH2−2×nO2.
At Standard Temperature and Pressure (STP), 1 mole of any gas occupies 22.4 L. Given volumes: VH2=9.08 L and VO2=2.27 L.
Moles of H₂: nH2=22.4VH2=22.49.08 mol. Moles of O₂: nO2=22.4VO2=22.42.27 mol.
Substitute these values into the equation for nH2S2O8: nH2S2O8=22.49.08−2×22.42.27 nH2S2O8=22.49.08−4.54 nH2S2O8=22.44.54 mol.
The molar mass of H₂S₂O₈ is approximately 194 g/mol. Mass of H₂S₂O₈ = nH2S2O8×Molar Mass Mass = 22.44.54×194≈39.32 g.
If NTP conditions (molar volume 22.7 L/mol) are used instead of STP (22.4 L/mol): nH2S2O8=22.74.54 mol. Mass = 22.74.54×194≈0.200×194=38.8 g. This matches option (c).
