Question
Question: Percentage weight of hydrogen in urea is: A. 6.67% B. 10% C. 4% D. 12.33%...
Percentage weight of hydrogen in urea is:
A. 6.67%
B. 10%
C. 4%
D. 12.33%
Solution
Hint: Solve this question by calculating the molecular weight of the compound using weights of the element. Then, divide the weight of the desired element by the molar mass of the compound to find weight of the compound in the element. Multiply it by 100 to find the percentage.
Complete step by step answer:
Urea, also known as carbamide, is a compound with molecular formula CO(NH2)2. It is a highly soluble organic compound formed in the liver from ammonia produced as a result of deamination of amino acids. It is a major organic component of human urine and is the waste product of many living organisms.
The structural formula of urea is –
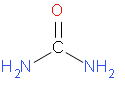
Let us start by calculating molar mass of urea.
= molar mass of carbon + molar mass of oxygen + (2 x molar mass of nitrogen) + (4 x molar mass of hydrogen)
= 12 g + 16 g + (2 x 14) g + (4 x 1) g
= 60 g.
% weight of element = molar mass of compoundmass of elementx 100 %
% weight of hydrogen =604 x 100 %
% weight of hydrogen = 6.67 %
Therefore, the answer is – option (a) – Percentage weight of hydrogen in urea is 6.67%.
Note: Percentage weights of other elements in urea are as follows –
Carbon
% weight = 6012 x 100 % = 19.99 %
Nitrogen
% weight = 6014 x 100 % = 46.64 %
Oxygen
% weight = 6016 x 100 % = 26.64 %
