Question
Question: A complex $CrCl_3, 6H_2O$ gives violet color solution in one isomeric form and grey color solution i...
A complex CrCl3,6H2O gives violet color solution in one isomeric form and grey color solution in another isomeric form These isomers are related as
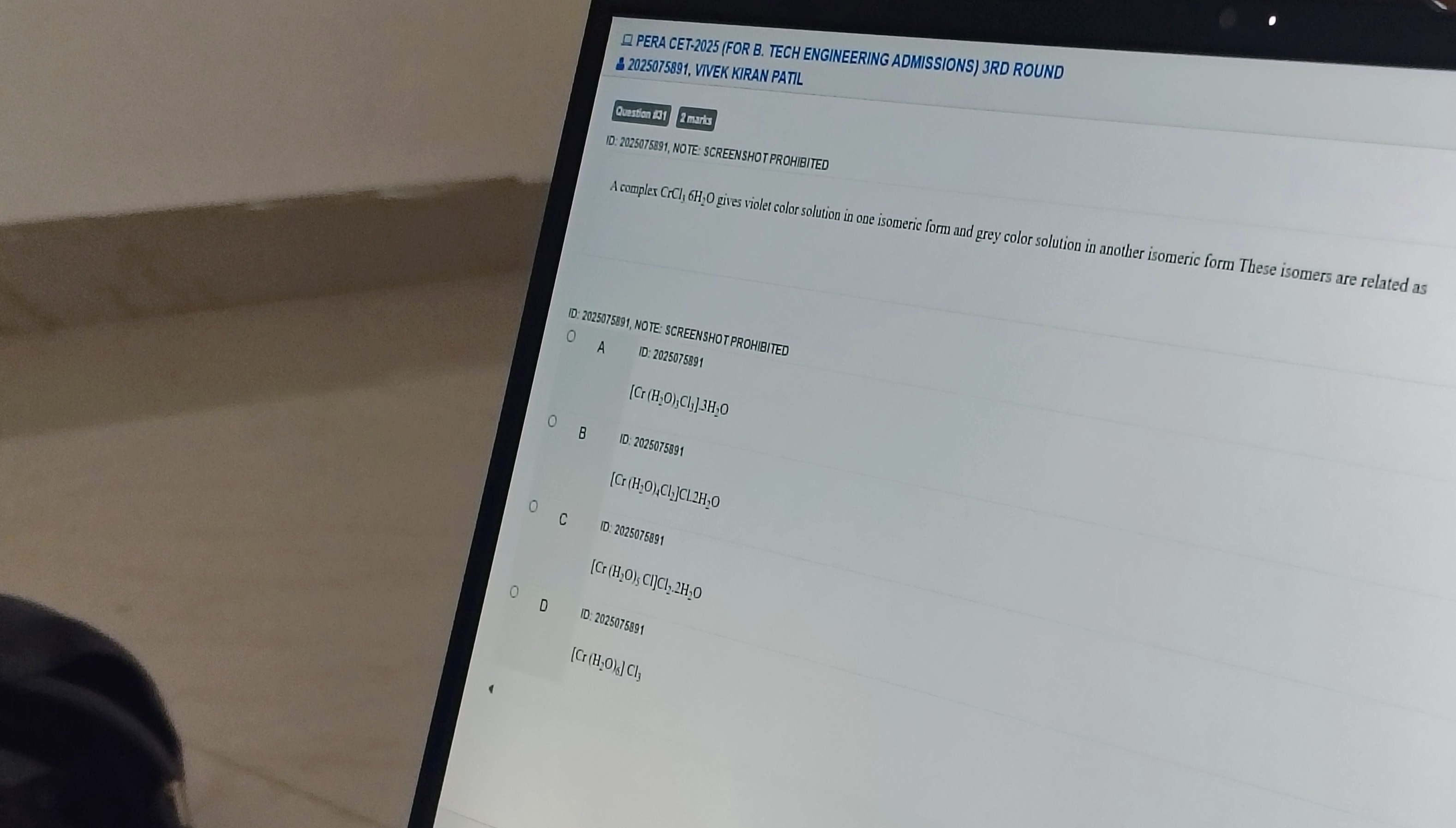
[Cr(H2O)3Cl3].3H2O
[Cr(H2O)4Cl2]Cl.2H2O
[Cr(H2O)5Cl]Cl2.2H2O
[Cr(H2O)6]Cl3
[Cr(H2O)6]Cl3
Solution
The complex CrCl3⋅6H2O exhibits hydration isomerism. The isomer [Cr(H2O)6]Cl3 is known to be violet, as all six water molecules are coordinated to the chromium ion. Other isomers, like [Cr(H2O)4Cl2]Cl⋅2H2O (dark green) and [Cr(H2O)3Cl3]⋅3H2O (green/grey-green), exist. Option D correctly identifies the violet isomer. Option C is incorrect as it contains 7 water molecules. Options A and B are valid isomers but represent the green/grey forms. Given that one form is violet and the options are specific formulas, the violet form (Option D) is the most distinct and primary candidate among the choices.
