Question
Question: \(PC{{l}_{5}}\) molecule has: A) Three fold axis of symmetry B) Two fold axis of symmetry C) B...
PCl5 molecule has:
A) Three fold axis of symmetry
B) Two fold axis of symmetry
C) Both A) and B)
D) None of these
Solution
The answer is dependent on the concept of the organic chemistry which deals with the stereochemistry part of a molecule and the answer is obtained by drawing the structure of the molecule and finding its hybridization.
Complete step by step answer:
- In the lower classes of chemistry, we have studied the basic concept of organic chemistry which includes the stereochemistry part and the related terms in it.
- Let us now see the stereochemistry of the given molecule so that we will be able to solve the given question.
- Stereochemistry is the branch of chemistry which deals with the study of a molecule in the imaginary three dimensional ways in a space and this is also called as the 3D chemistry. This gives the relationship between different molecules that are made of the same atoms.
To understand the base of the structure in space, we have to draw the structure of the given molecule.
- Now the structure of PCl5 according to the VSEPR theory is as shown below:
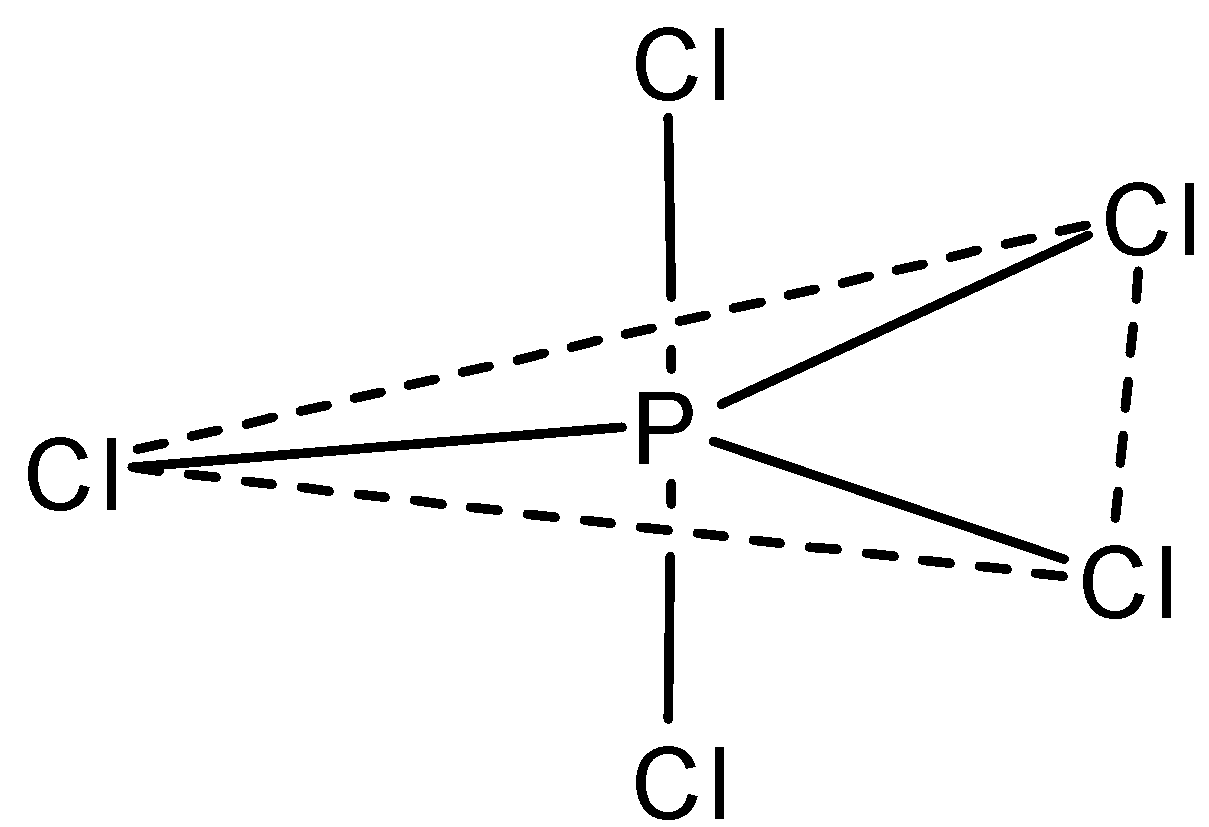
Here, the structure is trigonal bipyramidal with the three equatorial chlorine atoms having equivalent P−Cl bonds and the two axial bonds are longer than the equatorial bonds.
- The hybridization of this molecule is sp3 and therefore by these facts we can say that this molecule has both three fold axis and two fold axis of symmetry.
The correct option is option “C” .
Note: Note that the three fold axis in chemistry is nothing but the atoms attached to the central atom repeats themselves that is the molecule remains identical by the rotation of 1200 and they repeats three times in a 3600 rotation whereas two fold axis is when the molecule is identical after the rotation of 1800.
