Question
Question: \[PC{{l}_{3}}\] reacts with water, and then forms acid of phosphorus, what is the basicity of acid o...
PCl3 reacts with water, and then forms acid of phosphorus, what is the basicity of acid of phosphorus?
Solution
Hint: To answer this question, we should know that basicity of an acid is the number of replaceable hydrogen atoms present in a molecule of the acid. We should know that the acid which contains one, two, three replaceable hydrogen atoms in its molecule, they are called a monobasic, dibasic and tribasic acid respectively.
Step by step answer:
We should know that Phosphorous trichloride or phosphorous chloride is a colourless liquid. When we react it with water, it reacts violently with water to give the product of phosphoric acid and hydrochloride gas. It reacts violently with water and it produces an exothermic reaction involving release of acid gases.
PCl3+3H2O→H3PO4+3HCl
We should know that Phosphorous acid is a diprotic acid that is, it ionizes two protons. It is better described with the structural formula H3PO3 phosphorous acid is prepared by hydrolysis of phosphorus trichloride with acid or steam. Now, we should see the structure ofH3PO3.
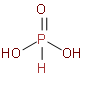
So, by observing the above structures we came to know that in its structure it has one double bond between P and O and two P and OH single bonds and one P and H bond. So, it has two acidic hydrogen that will come from a hydroxide molecule. The hydrogen which has a direct bond with phosphorus cannot be ionised so it is not acidic hydrogen. Hence we can say that only the H atoms attached with oxygen cause basicity. Only two hydrogens will come from the hydroxide molecule, so its basicity will be 2.
So, now we can say that phosphorous acid, H3PO3 is dibasic due to the presence of two P-OH bonds whereas phosphoric acid, H3PO4 is tribasic due to the presence of three P-OH bonds.
Note:
We should be careful in handling phosphorus trichloride. We should be careful because on exposure to skin it will cause severe burns. Inhalation of this compound leads to burning sensation, coughing. We should be careful when we want to handle large quantities ofPCl3. We should always work in dry surroundings, such as in a glove box. Avoid contact with water and humid environments.
