Question
Question: X₂/hv is used for free radical substitution reaction in alkanes R-H+X-X$\longrightarrow$R-X+H-X. The...
X₂/hv is used for free radical substitution reaction in alkanes R-H+X-X⟶R-X+H-X. The rate of halogens with alkanes follows as: F₂>Cl₂>Br₂>I₂. "Fluorination is too violent to be controlled" while Iodination is slow and reversible reaction. Relative rate of substitution of H-atoms of alkanes in free radical halogenation is: Cl₂⟹3°H : 2°H : 1°H 5 : 3.8 : 1 Br₂⟹3°H : 2°H : 1°H 1600 : 82 : 1 Isopentane reacts with Cl₂/hv to give a monochlorinated product P(major) and with Br₂/Δ to give a mono-brominated product Q(major).
alc. KOH (Q) ⟶ (T) ⟶ (U) Δ (Major) (Major) Q, T and U respectively are:
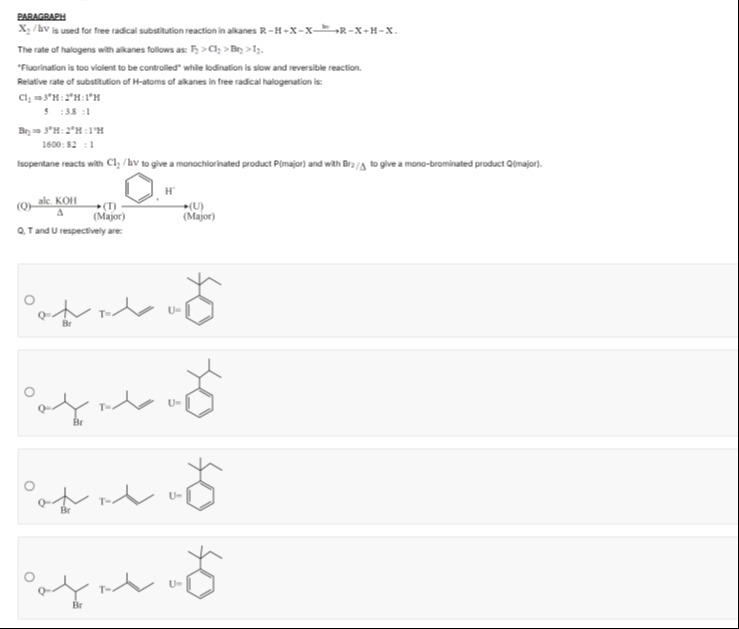
2-bromo-2-methylbutane, 2-methylbut-2-ene, 2-methyl-2-phenylbutane
1-bromo-2-methylbutane, 2-methylbut-1-ene, 2-methyl-1-phenylbutane
2-bromo-2-methylbutane, 2-methylbut-1-ene, 1-methyl-2-phenylbutane
1-bromo-2-methylbutane, 2-methylbut-2-ene, 1-methyl-1-phenylbutane
2-bromo-2-methylbutane, 2-methylbut-2-ene, 2-methyl-2-phenylbutane
Solution
Step-by-step Derivations:
-
Identify Isopentane: Isopentane is the common name for 2-methylbutane.
-
Determine Major Mono-brominated Product Q: Isopentane reacts with Br₂/Δ. This is a free radical substitution. The relative rates of substitution for Br₂ are given as 3°H : 2°H : 1°H = 1600 : 82 : 1.
Comparing these values (9 : 164 : 1600), the highest statistical reactivity is for the tertiary (3°) H-atom. Therefore, the major mono-brominated product Q will be formed by substitution at the tertiary carbon. Q is 2-bromo-2-methylbutane.
-
Determine Major Product T: Q (2-bromo-2-methylbutane) undergoes reaction with alc. KOH/Δ. This is a dehydrohalogenation (E2 elimination) reaction. The base (alc. KOH) removes a proton from a carbon adjacent to the carbon bearing the bromine (β-carbon), forming an alkene. According to Zaitsev's rule, the major product is the most substituted (more stable) alkene.
2-methylbut-2-ene is the more substituted and thus more stable alkene. Therefore, T (major) is 2-methylbut-2-ene.
-
Determine Major Product U: T (2-methylbut-2-ene) reacts with H⁺/Benzene. This is an acid-catalyzed Friedel-Crafts alkylation.
The tertiary carbocation (2-methylbutan-2-yl cation) is more stable and will be the predominant intermediate. This carbocation then acts as an electrophile and attacks the benzene ring. The product U will be 2-methyl-2-phenylbutane (also known as tert-pentylbenzene).
-
Conclusion:
- Q: 2-bromo-2-methylbutane
- T: 2-methylbut-2-ene
- U: 2-methyl-2-phenylbutane
